Preparation method of azithromycin for injection
A technology for azithromycin and injection, which is applied in the field of medicine, can solve the problems of high waste rate and low yield of azithromycin, and achieve the effects of reduced waste rate, good stability and reconstitution rate, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The invention provides a preparation method of azithromycin for injection, comprising the following steps:
[0015] Step 1, adding co-solvent and azithromycin in sequence to the water for injection, adding a pH regulator until the pH is 6.5-6.8, constant volume, filtering and sterilizing to obtain a sterile azithromycin solution;
[0016] Step 2, filling and half-stoppering the sterile azithromycin solution, and pre-freezing at -70°C for 2 hours;
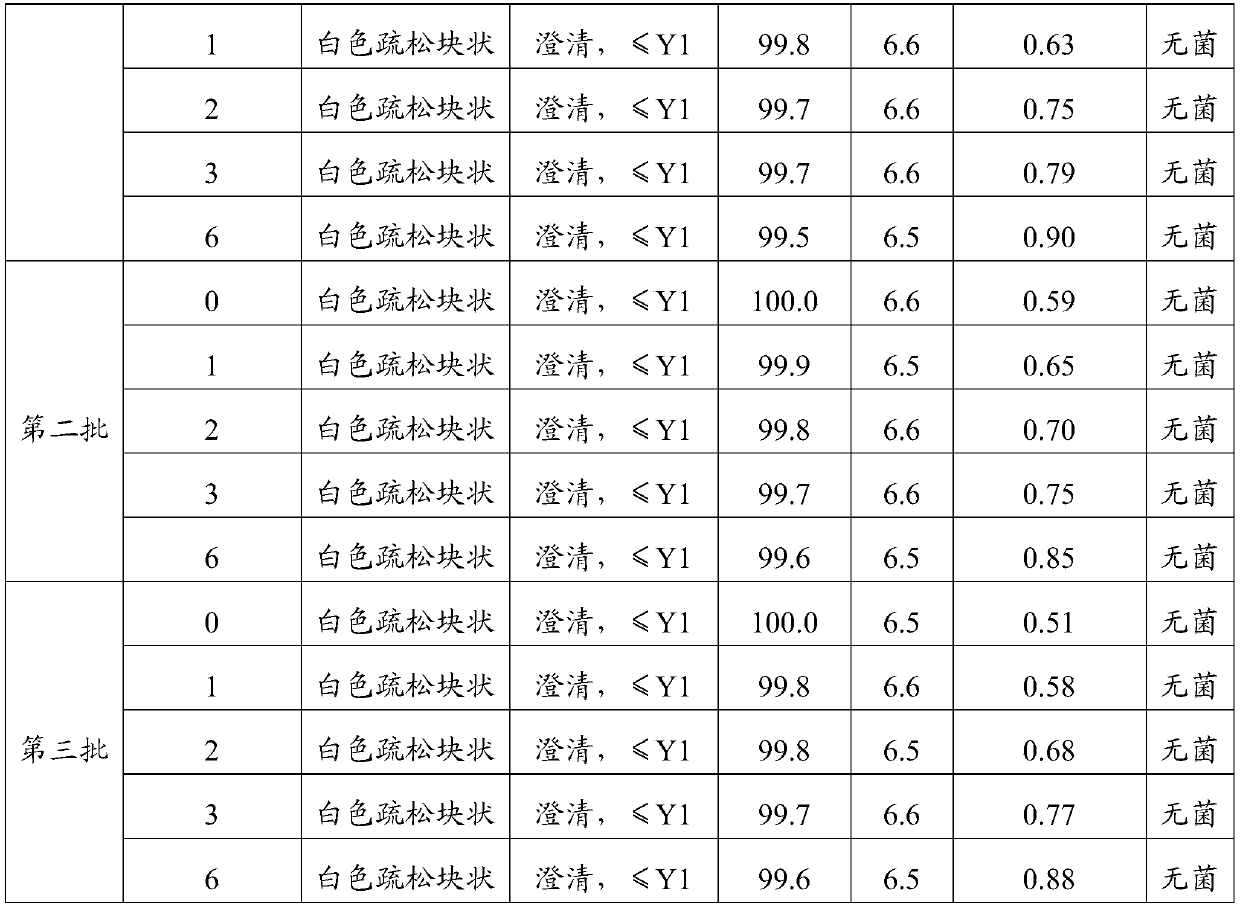
[0017] Step 3. After pre-freezing in step 2, carry out five-stage sublimation drying, first-level: temperature -20--10°C, vacuum degree 10-100mbar, keep for 3h; second-level: temperature-10-0°C, vacuum degree 10- 100mbar, keep for 5h; level 3: temperature 0℃, vacuum 100-200mbar, keep 5h; level 4: temperature 5-15℃, vacuum 150-200mbar, keep 6h; level 5: temperature 10-25℃, vacuum 50-150mbar, keep for 3h;
[0018] Among them, during the sublimation drying process, the temperature showed an upward trend, and the vacuum degree ...
Embodiment 1
[0031] The preparation method of azithromycin for injection specifically comprises the following steps:
[0032] Step 1. Add 4g glutamic acid and 1.024g citric acid to 800mL water for injection at 20°C to dissolve, then add 12.5g azithromycin and stir until dissolved, add sodium bicarbonate to pH 6.8, dilute to 1000mL, then add 0.1 % (g / ml) of activated carbon, stirred and decolorized, then decarbonized and filtered with a 0.45 μm filter membrane, and then sterilized and filtered with a 0.22 μm filter membrane to obtain a sterile azithromycin solution;
[0033] Step 2. Cool the sterile azithromycin solution to 10°C, perform filling and semi-stoppering, and pre-freeze at -70°C for 2 hours under normal pressure, and the cooling rate to the pre-freezing temperature is 28°C / h;
[0034] Step 3. After the pre-freezing in step 2, carry out five-stage sublimation drying. The first stage: temperature -20°C, vacuum degree 100mbar, keep for 3h; the second stage: temperature 0°C, vacuum d...
Embodiment 2
[0037] The preparation method of azithromycin for injection specifically comprises the following steps:
[0038] Step 1. Add 2.75g of glutamic acid and 1.125g of citric acid to 750mL of water for injection at 30°C to dissolve, then add 12.5g of azithromycin and stir until dissolved, add sodium bicarbonate until the pH is 6.8, set the volume to 1000mL, and then add 0.1% (g / ml) of activated carbon, stirred and decolorized, then decarbonized and filtered with a 0.45 μm filter membrane, and then sterilized and filtered with a 0.22 μm filter membrane to obtain a sterile azithromycin solution;
[0039] Step 2. Cool the sterile azithromycin solution to 4°C, perform filling and half stoppering, and pre-freeze at -70°C for 2 hours under normal pressure, and the cooling rate to the pre-freezing temperature is 35°C / h;
[0040] Step 3. After the pre-freezing in step 2, carry out five-stage sublimation drying. The first stage: temperature -20°C, vacuum degree 100mbar, keep for 3h; the seco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com