Azithromycin detection molecular imprinting monolithic micro column and preparation method thereof
A molecular imprinting and azithromycin technology, applied in the field of material chemistry, can solve the problems affecting the sensitivity and accurate quantification of detection methods, strong matrix effect, lack of selectivity, etc., to overcome cumbersome pretreatment, high selectivity and specificity, high affinity. Peaceful and selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
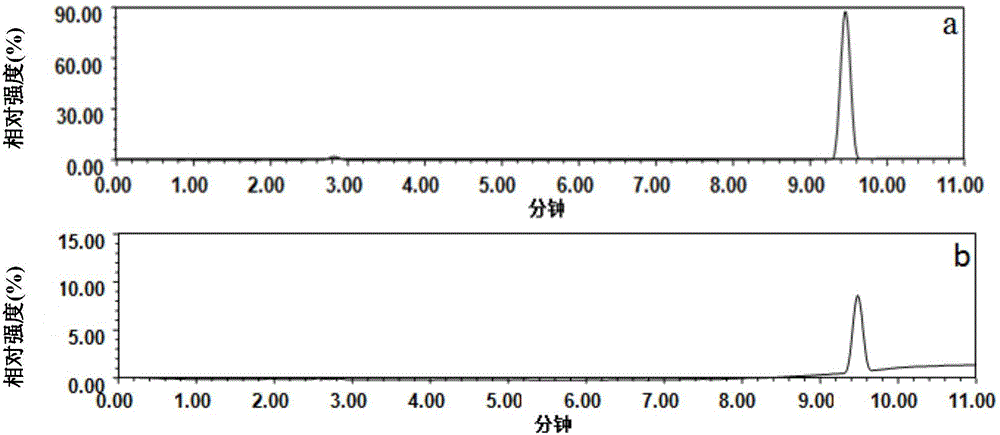
[0046] (1) Weigh 0.034g (0.04mmoL) spiramycin into a test tube, add 500μL of a mixed porogen of toluene and dodecanol (1:4, v / v), vortex to dissolve, then add 13.6μL ( 0.16mmoL) methacrylic acid functional monomer, ultrasonic for 5min, and stand for 1h to form a prepolymer.
[0047] (2) Add 150μL (0.8mmoL) of ethylene glycol dimethacrylate and 2mg (0.012mmol) of azobisisobutyronitrile to the above-mentioned prepolymer, sonicate for 2min, blow nitrogen gas for 5min, and pipette 40μL into a sealed container at one end. sealed in a small pipette tip, and polymerized in a vacuum oven at 60°C for 24 hours to obtain a small pipette tip for imprinted micro-extraction.
[0048] (3) Seal both ends of the above-mentioned small pipette tip, connect a 2.5mL syringe, load it on a screw syringe pump, and wash it with 6mL of a mixed solution of acetic acid and methanol (the volume ratio of acetic acid and methanol is 1:9, v / v) The template spiramycin was removed, and the flow rate was set t...
Embodiment 2
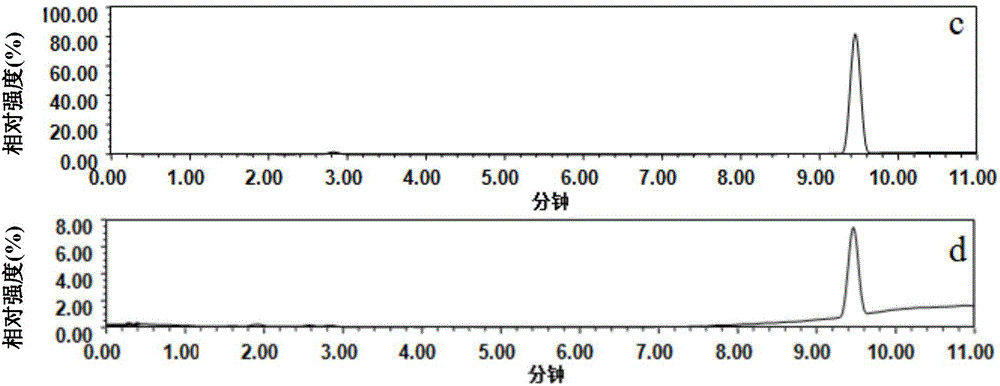
[0052] (1) Weigh 0.034g (0.04mmoL) tilmicosin into a test tube, add 600μL toluene / dodecanol (1:4, v / v) mixed porogen, vortex to dissolve, then add 13.6μL (0.16mmoL) methacrylic acid functional monomer, sonicated for 5min, and allowed to stand for 1h to form a prepolymer.
[0053] (2) Add 150μL (0.8mmoL) of ethylene glycol dimethacrylate and 2mg (0.012mmol) of azobisisobutyronitrile to the above-mentioned prepolymer, sonicate for 2min, blow nitrogen gas for 5min, and pipette 40μL into a sealed container at one end. sealed in a small pipette tip, and polymerized in a vacuum oven at 60°C for 24 hours to obtain a small pipette tip for imprinted micro-extraction.
[0054] (3) Seal both ends of the above-mentioned small pipette tip, connect a 2.5mL syringe, load it on a screw syringe pump, wash with 6mL of a mixed solution of acetic acid and methanol (the volume ratio of acetic acid and methanol is 1:9) to remove template spiral mold The flow rate was 0.05mL / min, and then washed wi...
Embodiment 3
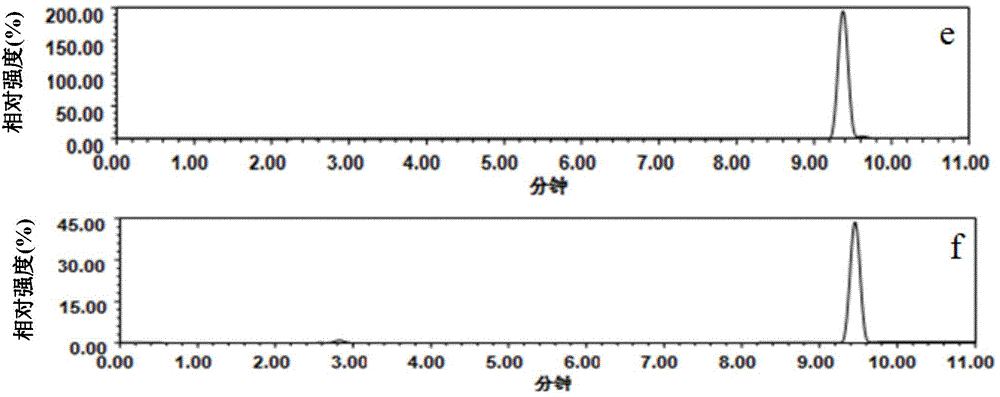
[0058] (1) Weigh 0.034g (0.04mmoL) spiramycin into a test tube, add 500μL of a mixed porogen of toluene and dodecanol (1:4, v / v), vortex to dissolve, then add 13.6μL ( 0.16mmoL) p-vinylbenzoic acid functional monomer, ultrasonic for 5min, and stand for 1h to form a prepolymer.
[0059] (2) Add 150.4μL (0.8mmoL) of ethylene glycol dimethacrylate and 2mg (0.012mmol) of azobisisobutyronitrile to the above prepolymer, sonicate for 2min, blow nitrogen gas for 5min, pipette 40μL and seal at one end sealed, and polymerized in a vacuum oven at 60°C for 24 hours to obtain imprinted micro-extraction tips.
[0060] (3) Seal both ends of the above-mentioned small pipette tip, connect a 2.5mL syringe, load it on a screw syringe pump, and wash it with 6mL of a mixed solution of acetic acid and methanol (the volume ratio of acetic acid and methanol is 1:9, v / v) The template spiramycin was removed at a flow rate of 0.05 mL / min, and acetic acid was removed by washing with 6 mL of methanol at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com