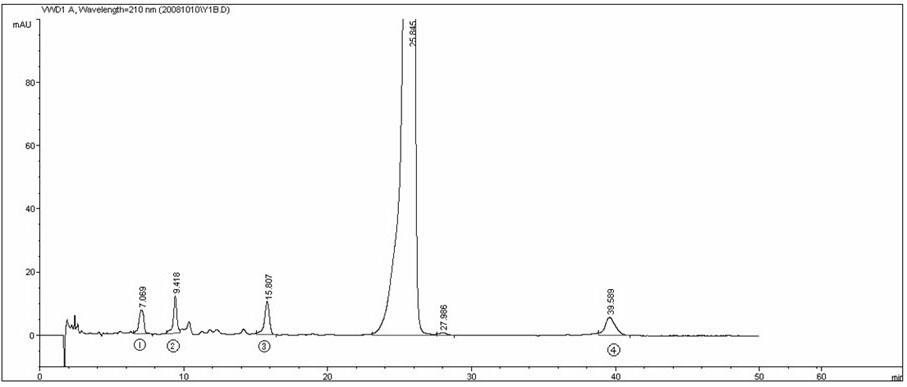
Stabilizer of lyophilized powder injection for azithromycin injection
A kind of freeze-dried powder injection, azithromycin technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Prescription content: Azithromycin: 26.935g
[0034] Citric acid: 8.269g
[0035] Sodium hydroxide: 1.753g
[0036] Preparation process: Dissolve 26.935g of azithromycin, 8.2769g of citric acid, and 1.753g of sodium hydroxide in water, stir, filter, and freeze-dry.
[0037] Stability test results:
[0038] Test sample Red mold (A)% Archie GX% Archie B% Simple% Total Miscellaneous% raw material 0.078 0.000 0.273 0.128 0.78 finished product 0.052 0.000 0.278 0.181 0.76 5 days 0.062 0.02 0.281 0.214 1.01 10 days 0.066 0.024 0.303 0.140 1.16 plus 1 0.065 0.027 0.309 0.133 1.02 plus 2 0.078 0.028 0.299 0.126 1.04 plus 3 0.080 0.021 0.298 0.154 1.12 plus 6 0.110 0.026 0.304 0.191 1.48 cool 1 0.045 0.025 0.285 0.154 0.73 cool 2 0.054 0.021 0.343 0.115 0.81 cool 3 0.054 0.022 0.352 0.176 0.94 cool 6 0.067 0...
Embodiment 2
[0040] Prescription content: Azithromycin: 26.924g
[0041] Citric acid: 17.607g
[0042] Sodium hydroxide: 7.228g
[0043] Preparation process: Weigh 26.924g of azithromycin, 17.607g of citric acid, and 7.228g of sodium hydroxide, dissolve them in water, stir, filter, and freeze-dry.
[0044] Stability test results:
[0045] Test sample Red mold (A)% Archie GX% Archie B% Simple% Total Miscellaneous% raw material 0.078 0.000 0.273 0.128 0.78 finished product 0.048 0.011 0.319 0.131 0.70 5 days 0.065 0.020 0.267 0.182 0.91 10 days 0.103 0.022 0.303 0.151 1.21 plus 1 0.066 0.032 0.299 0.145 1.02 plus 2 0.068 0.033 0.310 0.133 1.01 plus 3 0.079 0.021 0.322 0.157 1.16 plus 6 0.119 0.023 0.314 0.216 1.47 cool 1 0.051 0.028 0.310 0.133 0.74 cool 2 0.056 0.021 0.289 0.113 0.78 cool 3 0.058 0.024 0.346 0.161 0.87 coo...
Embodiment 3
[0047] Prescription content: Azithromycin: 26.934g
[0048] Citric acid: 21.475g
[0049] Sodium hydroxide: 9.517g
[0050] Preparation process: Weigh 26.934g of the main ingredient, 21.475g of citric acid, and 9.517g of sodium hydroxide, dissolve them in water, stir, filter, and freeze-dry.
[0051] Stability test results:
[0052] Test sample Red mold (A)% Archie GX% Archie B% Simple% Total Miscellaneous% raw material 0.078 0.000 0.273 0.128 0.78 finished product 0.074 0.000 0.268 0.161 0.79 5 days 0.074 0.000 0.287 0.176 0.91 10 days 0.107 0.022 0.264 0.150 1.11 plus 1 0.072 0.029 0.288 0.147 0.97 plus 2 0.085 0.026 0.326 0.120 1.02 plus 3 0.089 0.023 0.333 0.143 1.07 plus 6 0.115 0.023 0.310 0.193 1.43 cool 1 0.063 0.021 0.298 0.116 0.74 cool 2 0.065 0.020 0.294 0.111 0.79 cool 3 0.065 0.024 0.301 0.142 0.85 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com