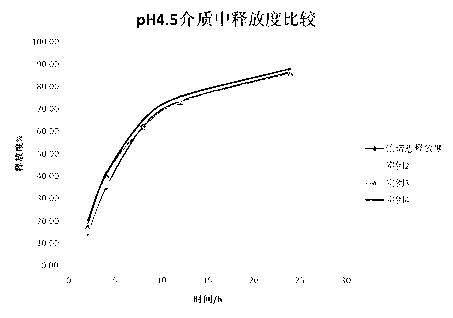
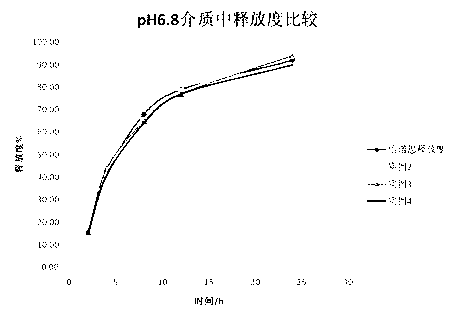
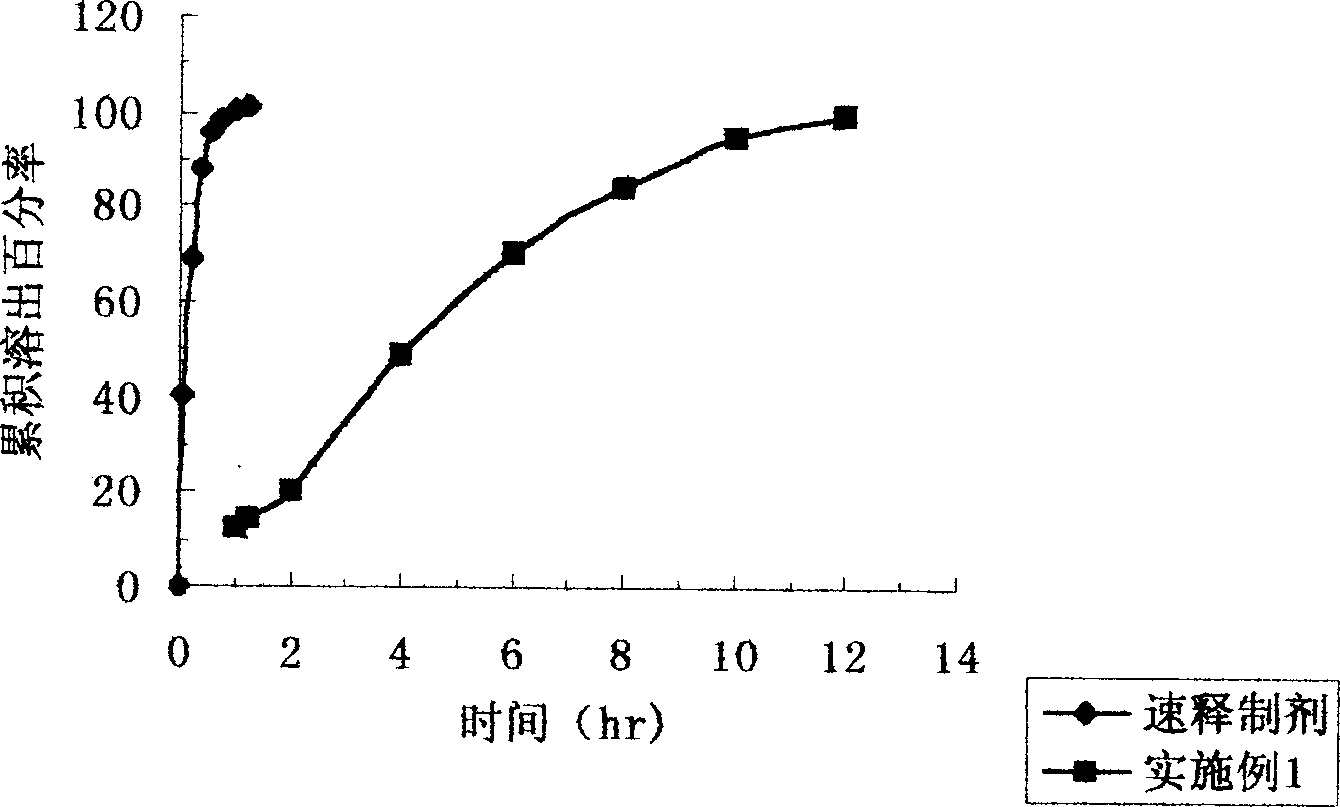
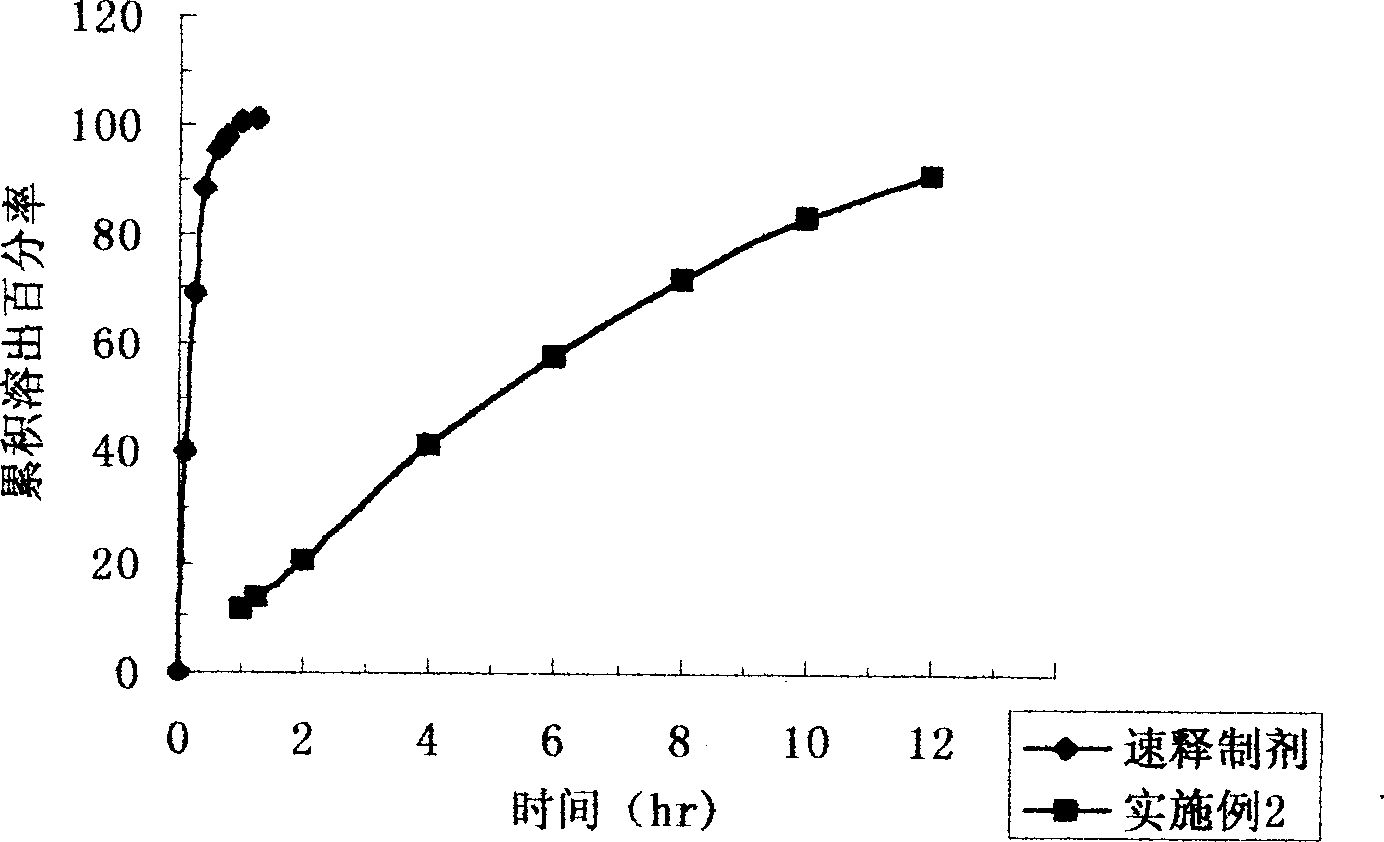
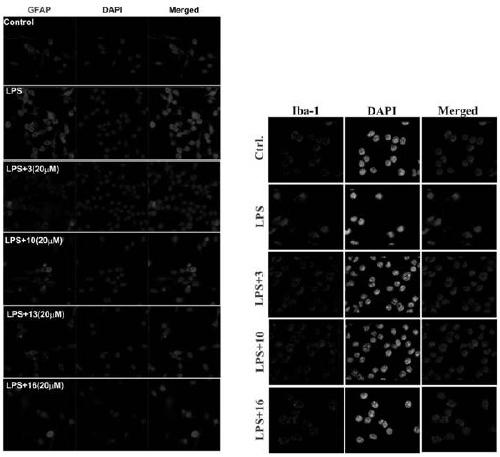
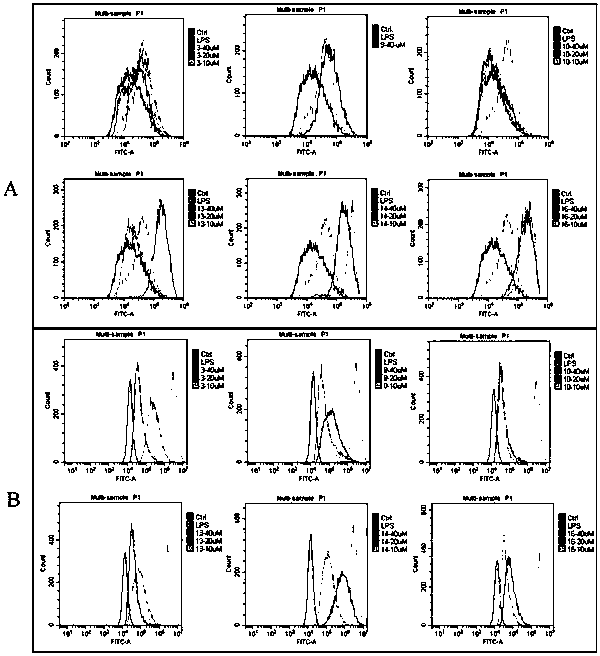
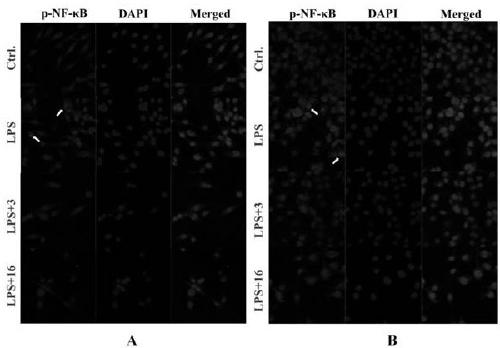
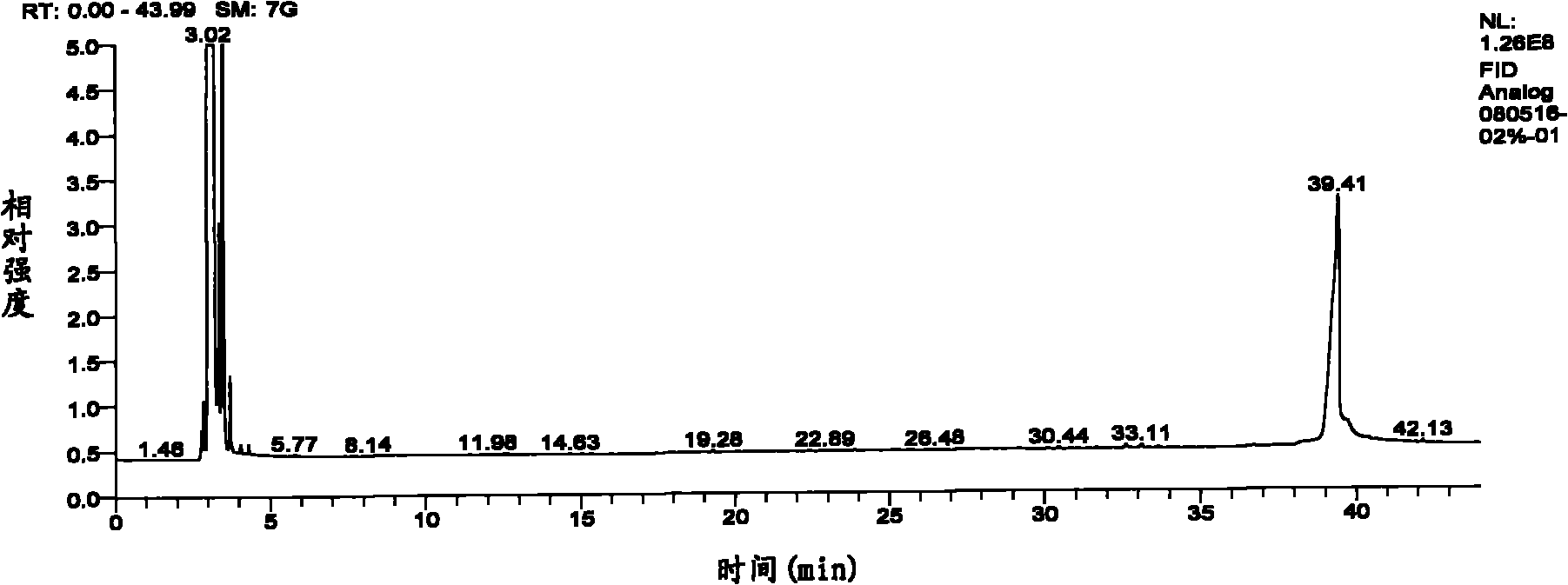
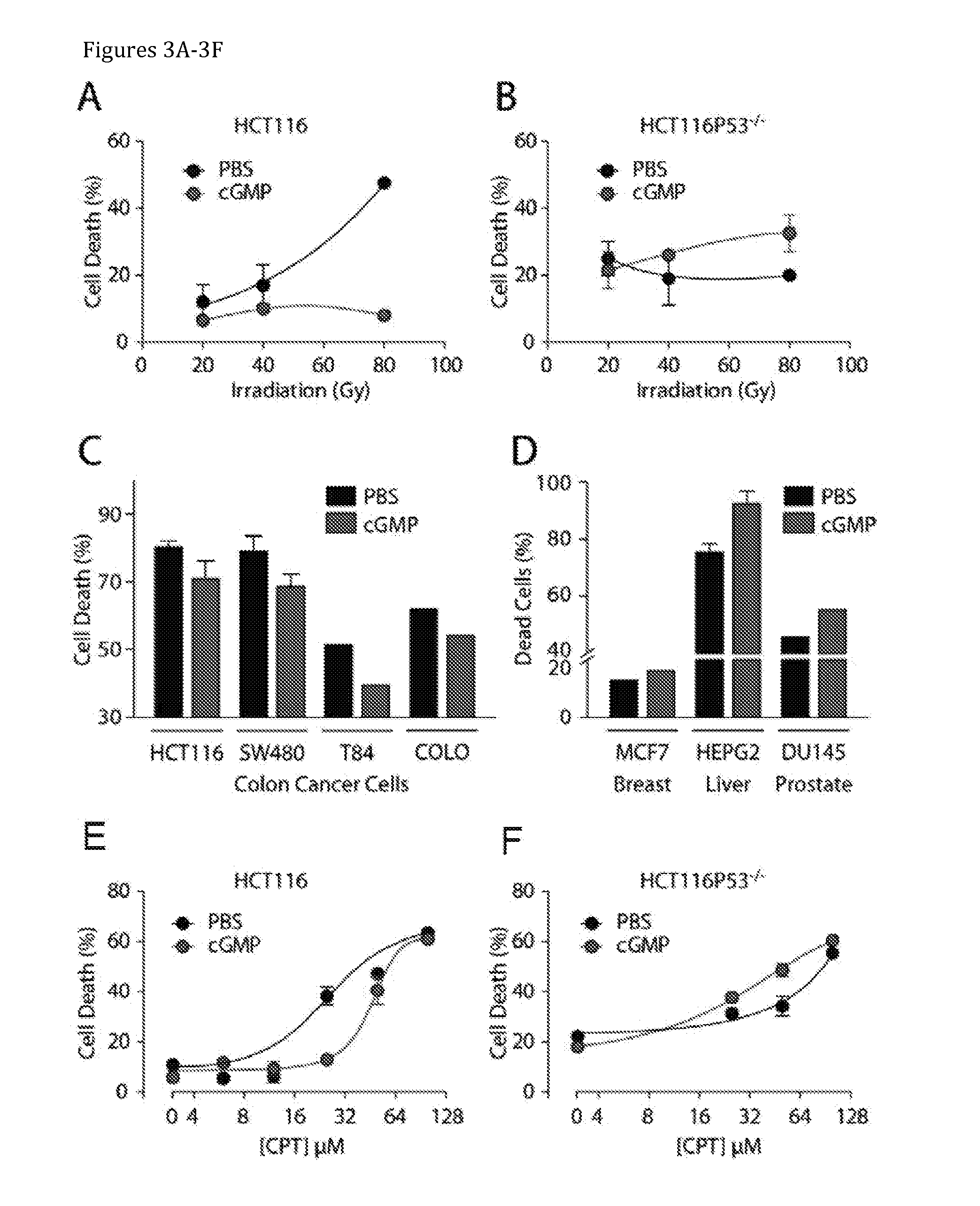
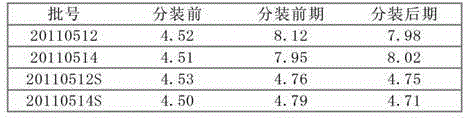
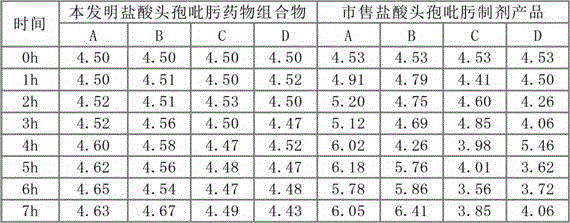
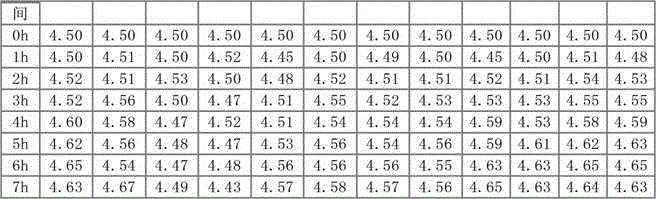
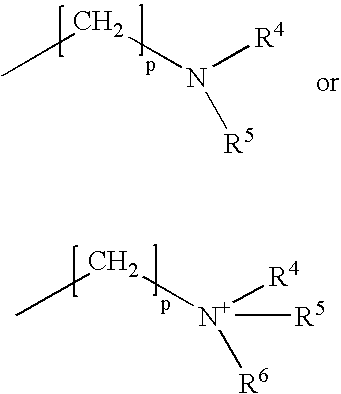
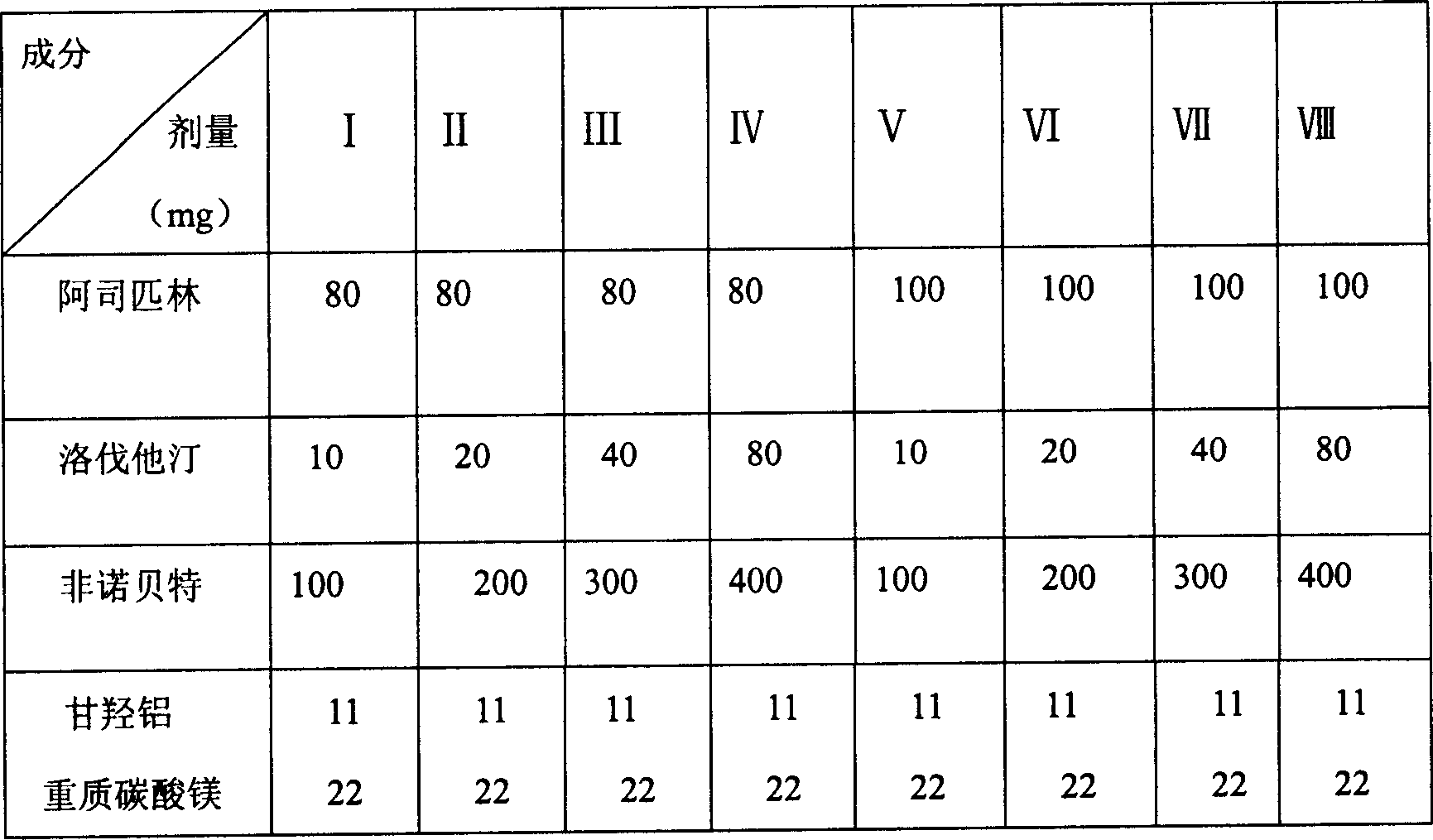
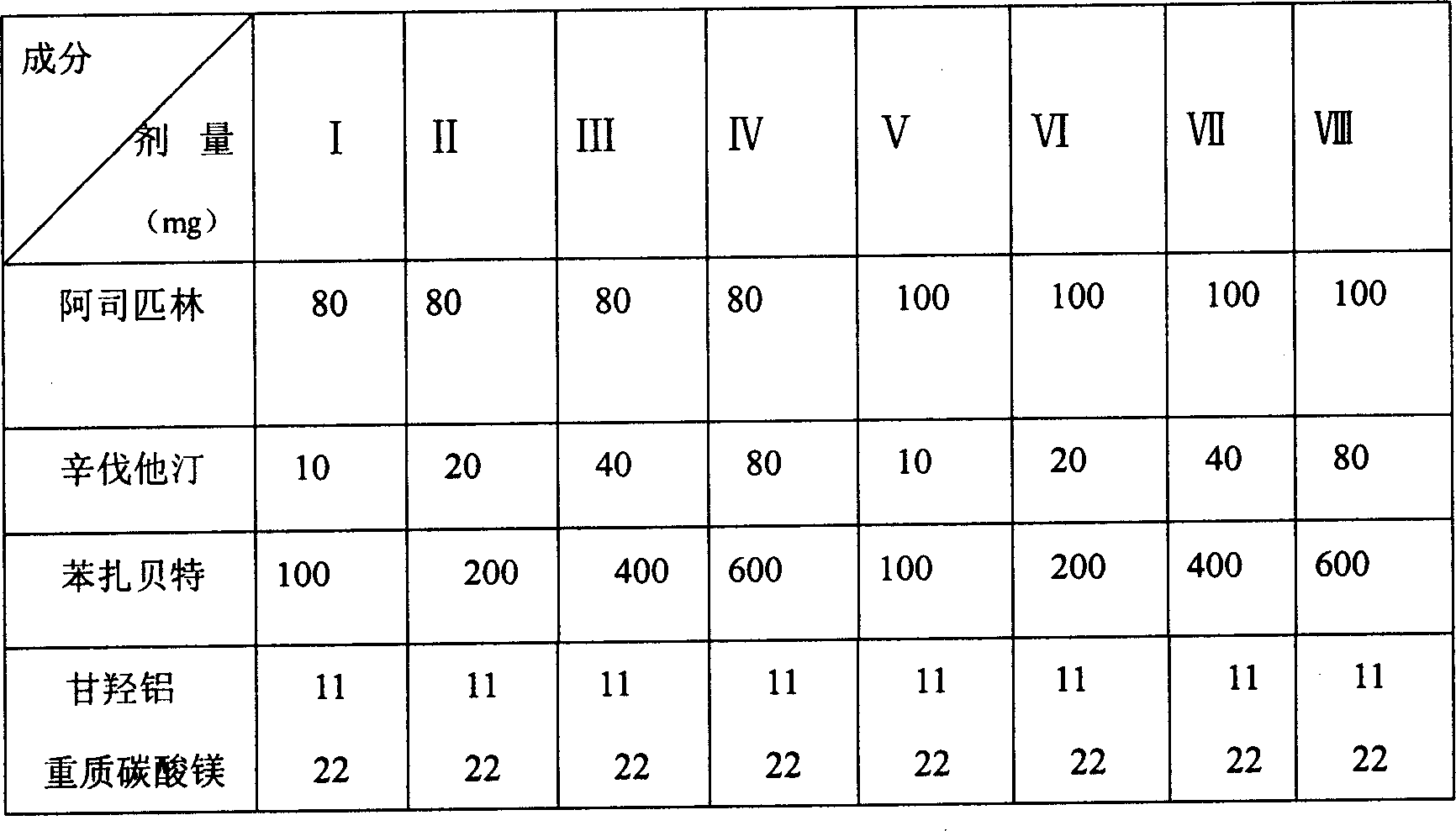
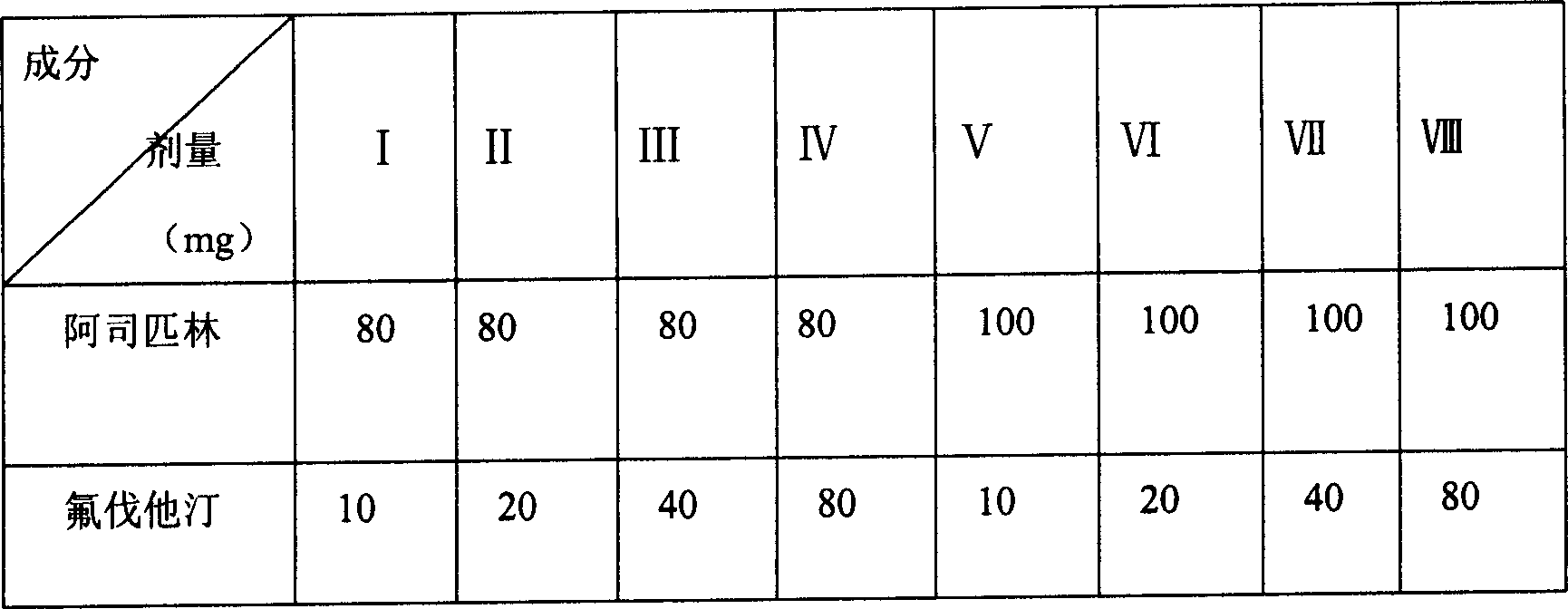
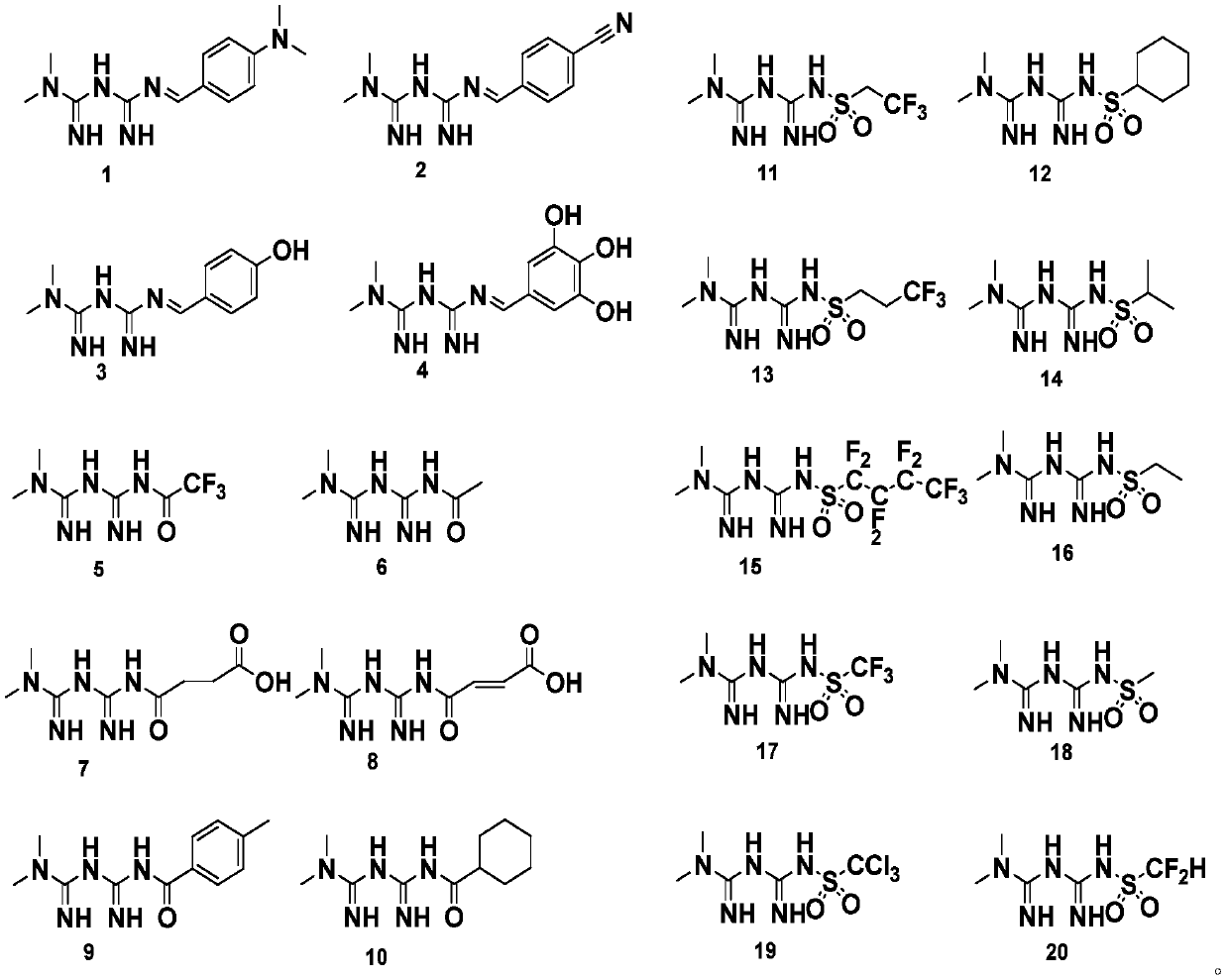
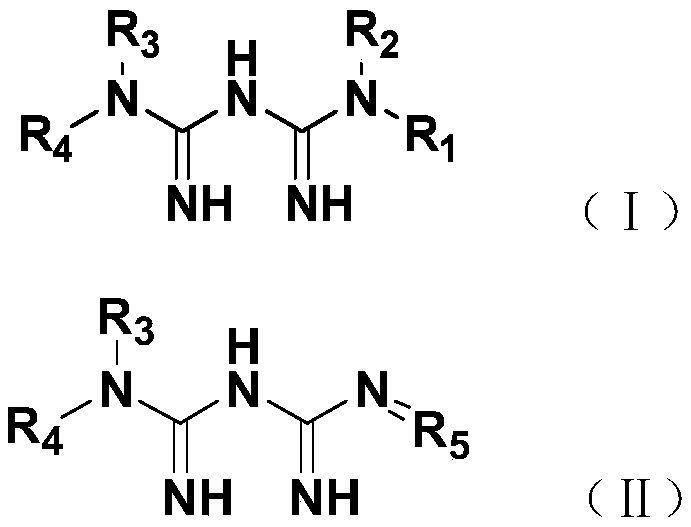
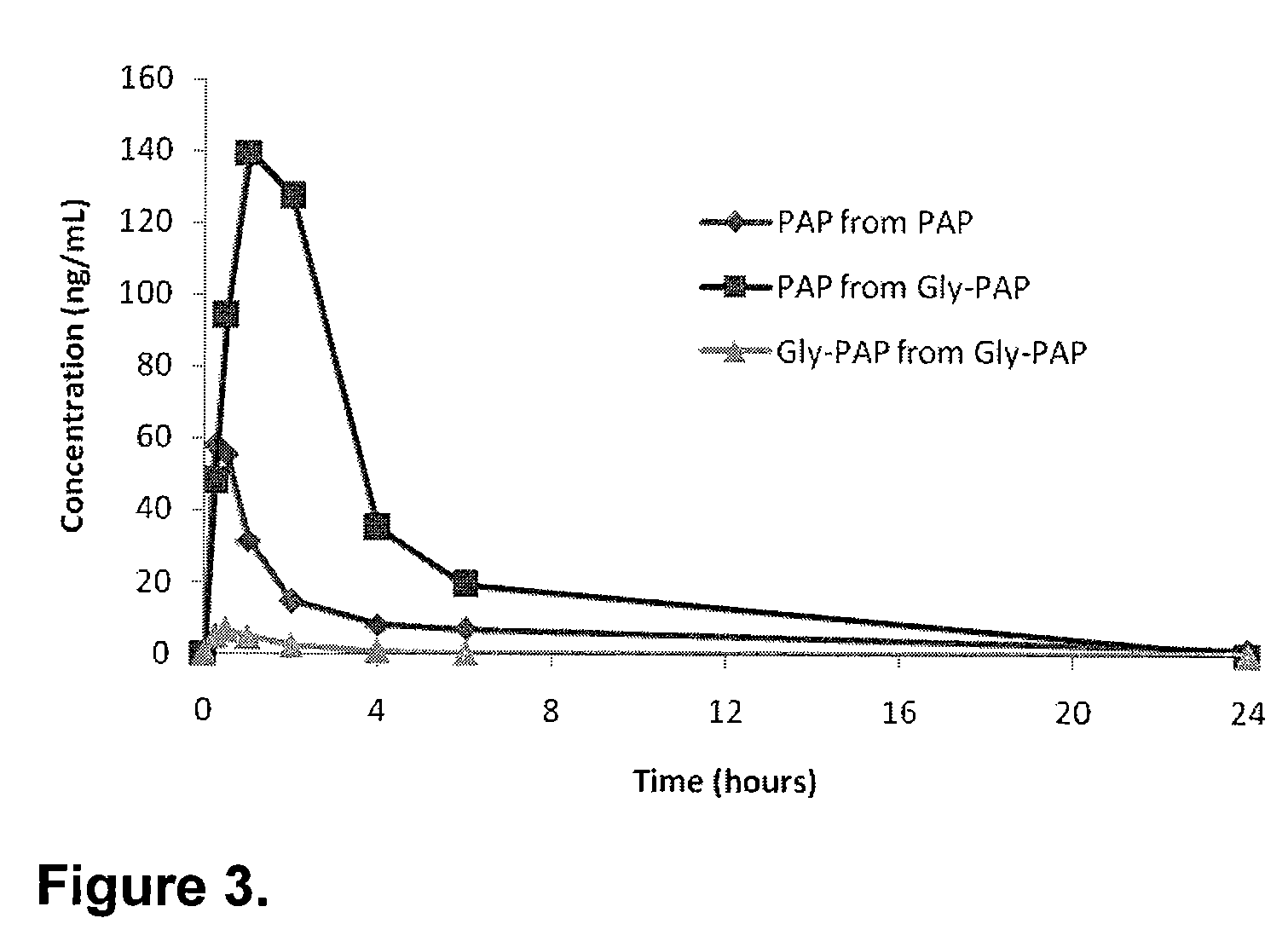
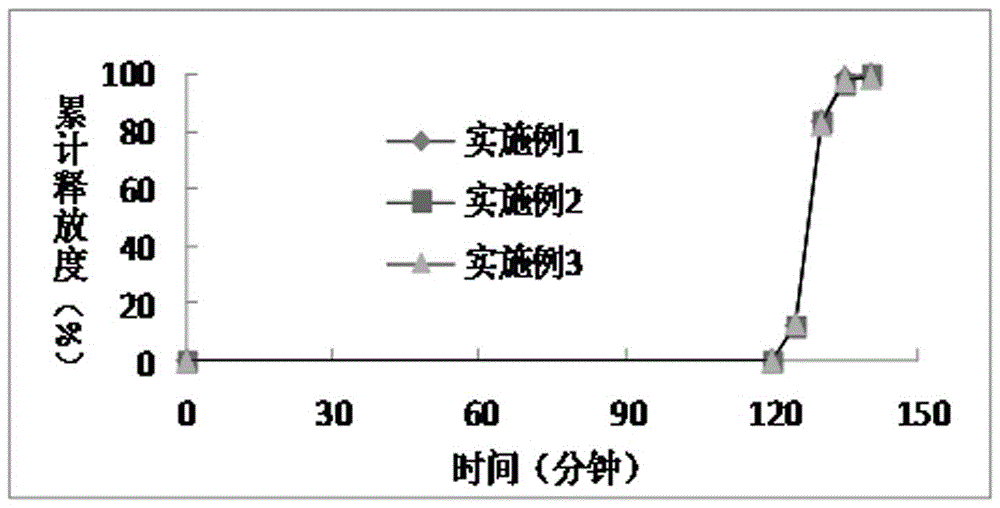
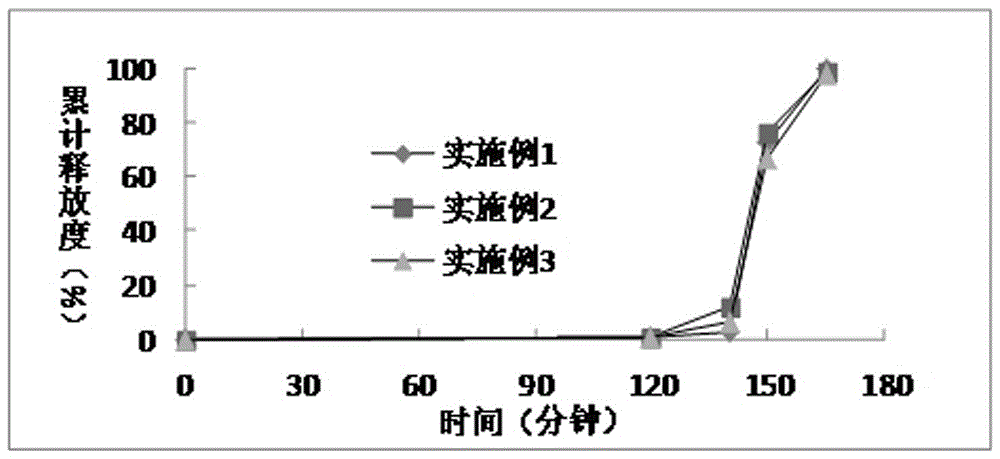
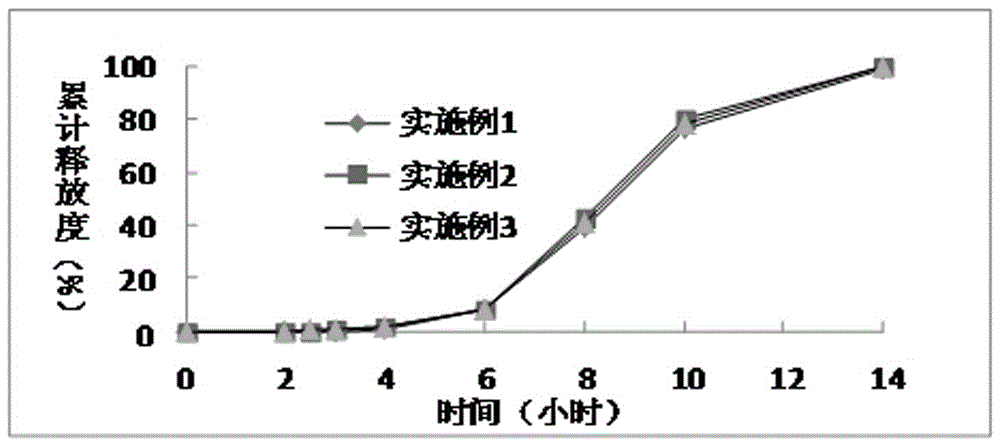
Patents
Literature
62results about How to "Reduce gastrointestinal side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
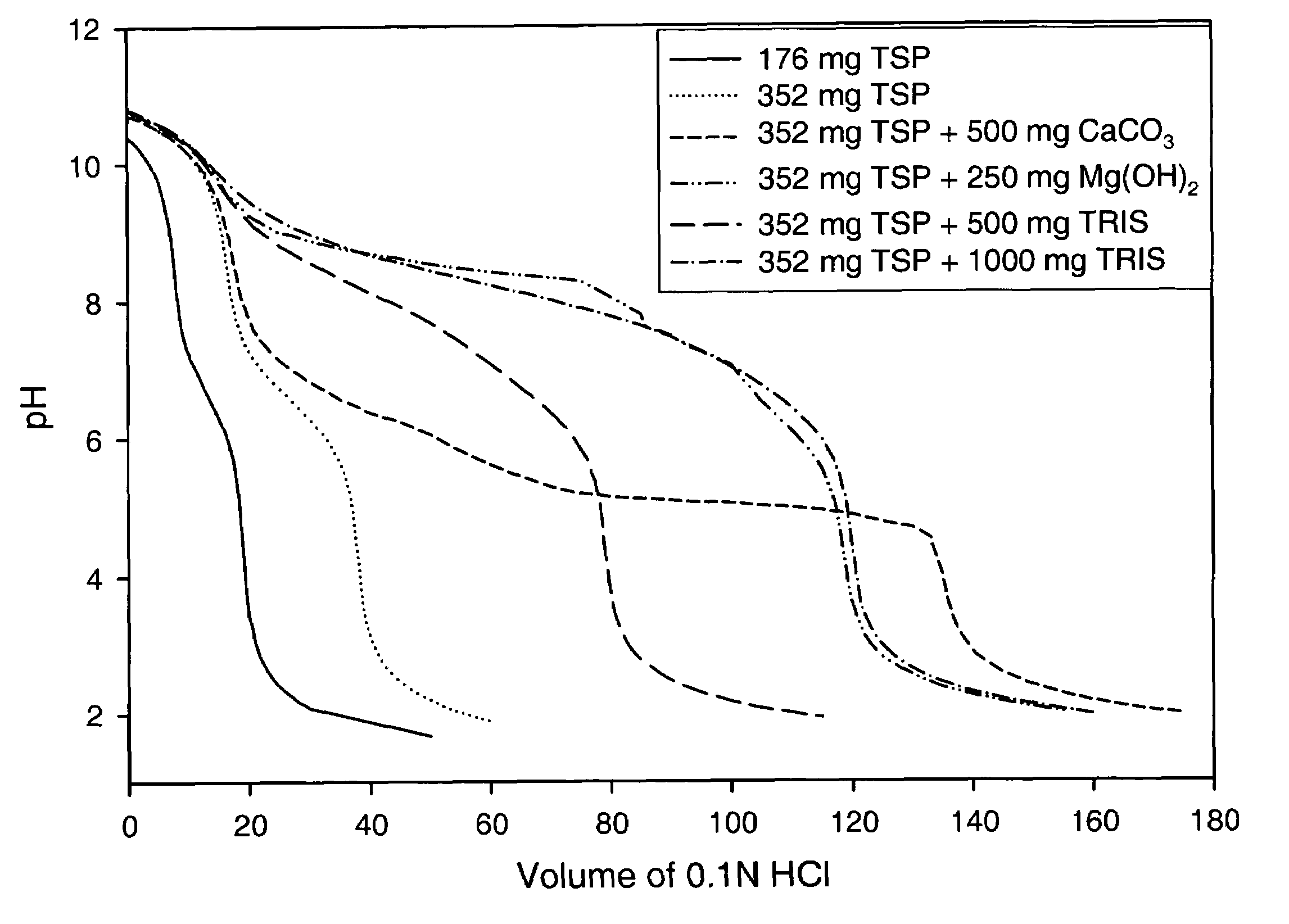
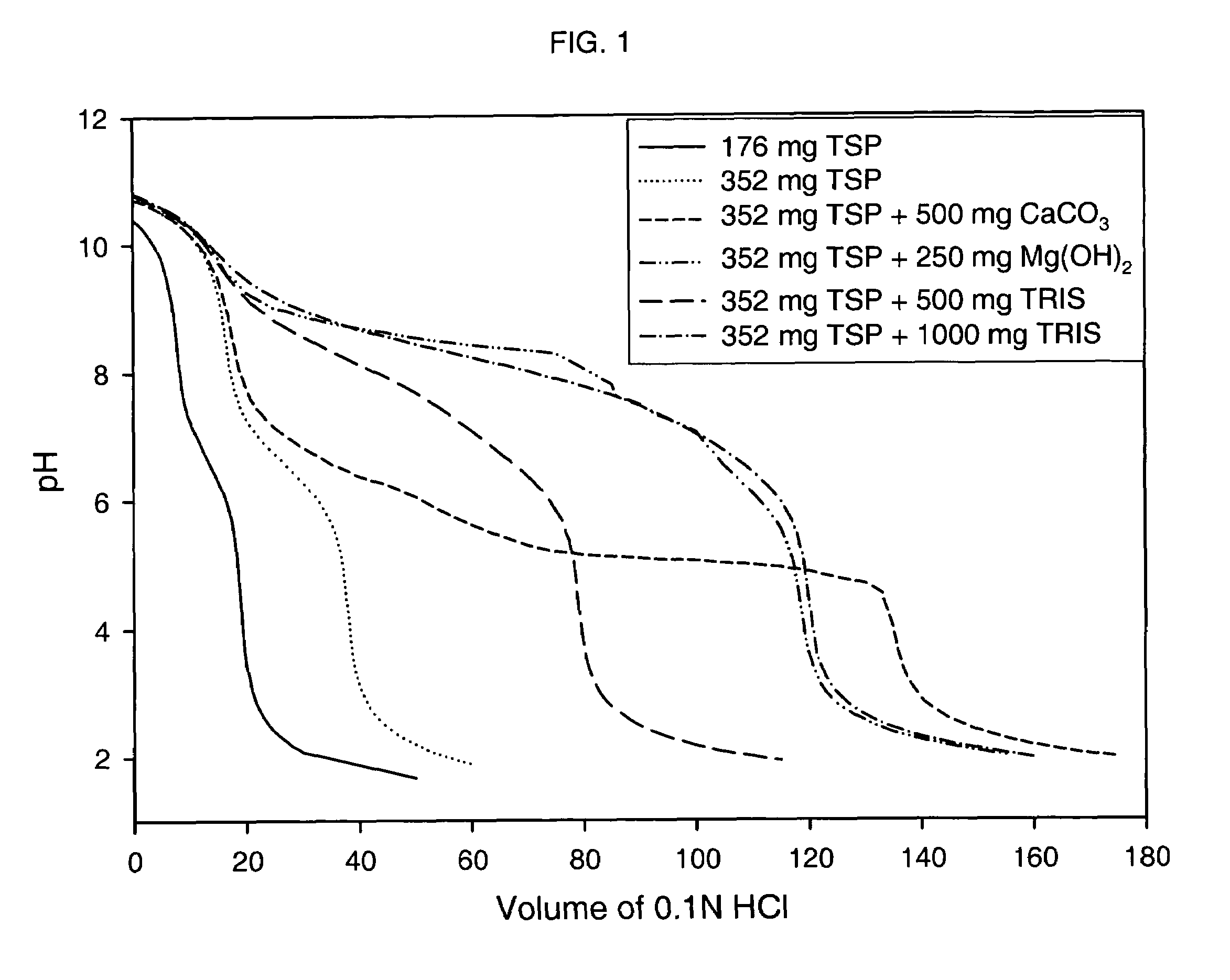
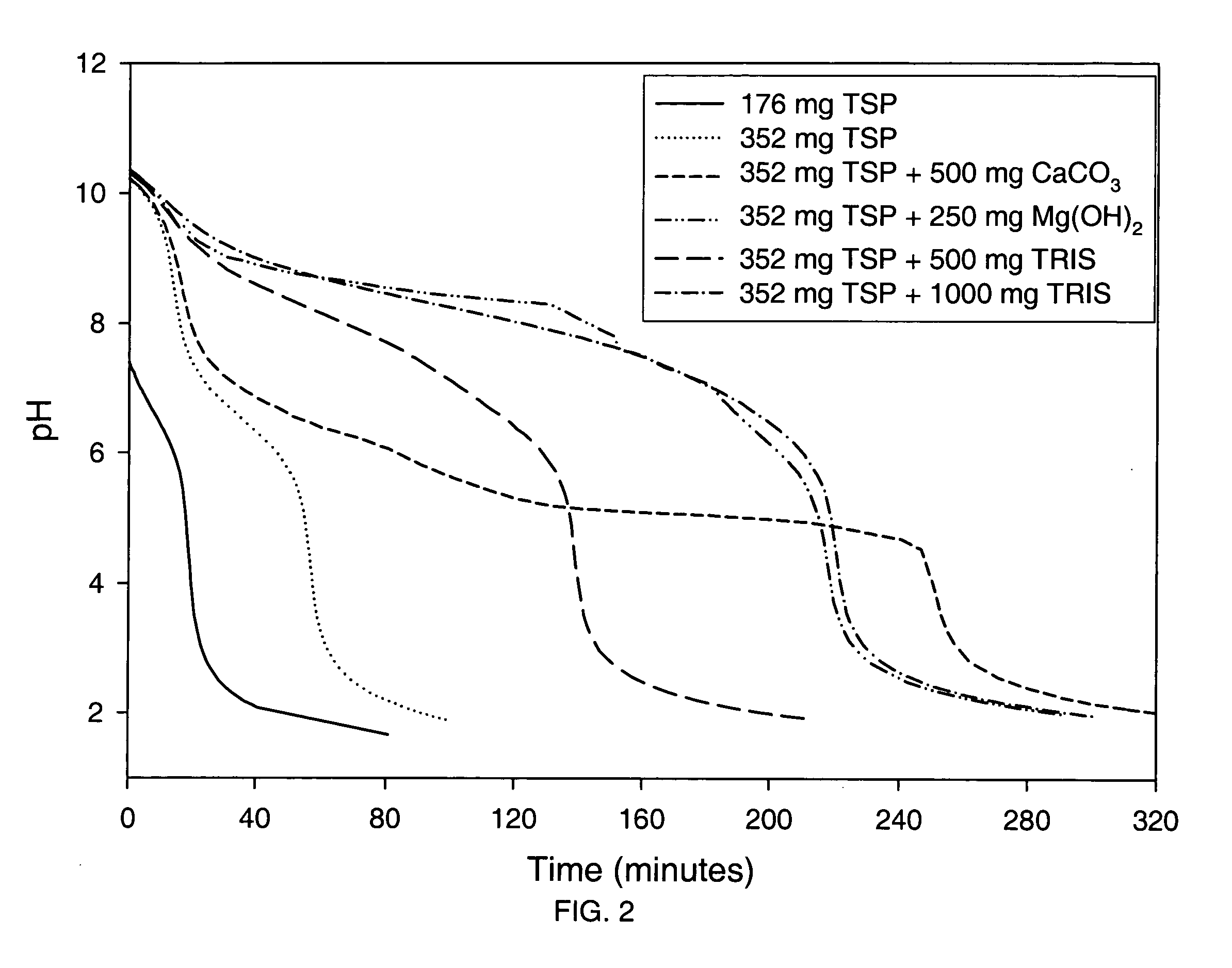
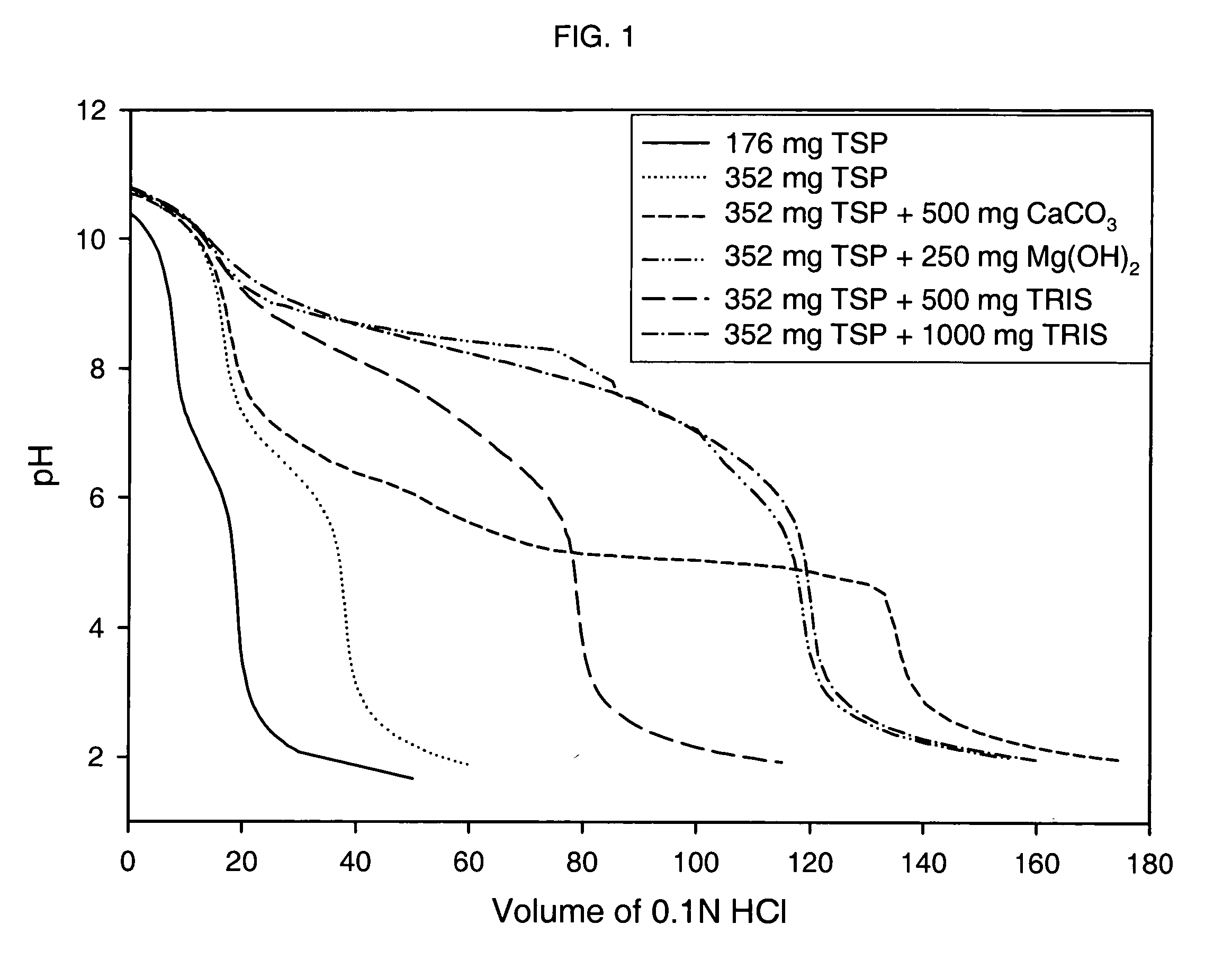
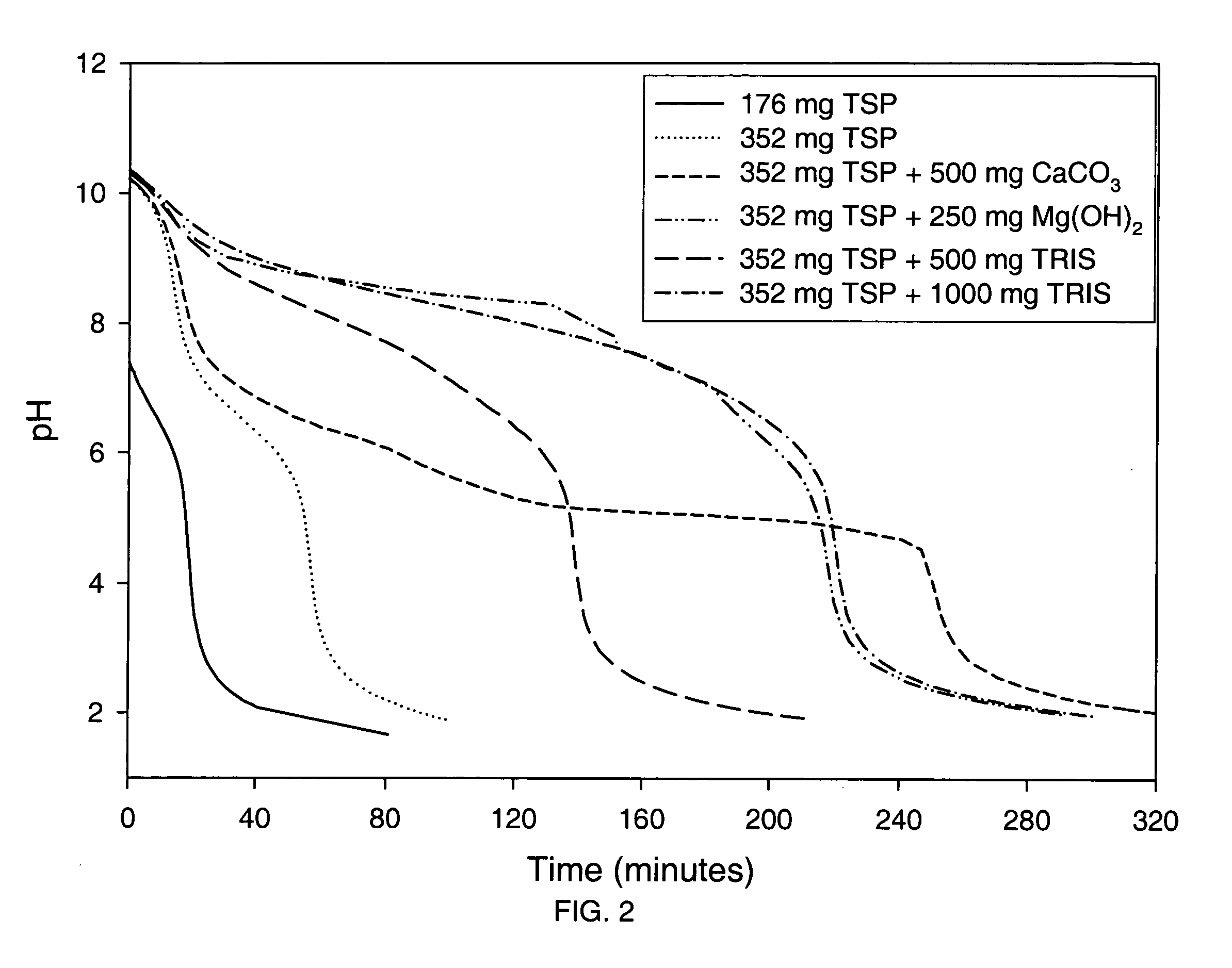
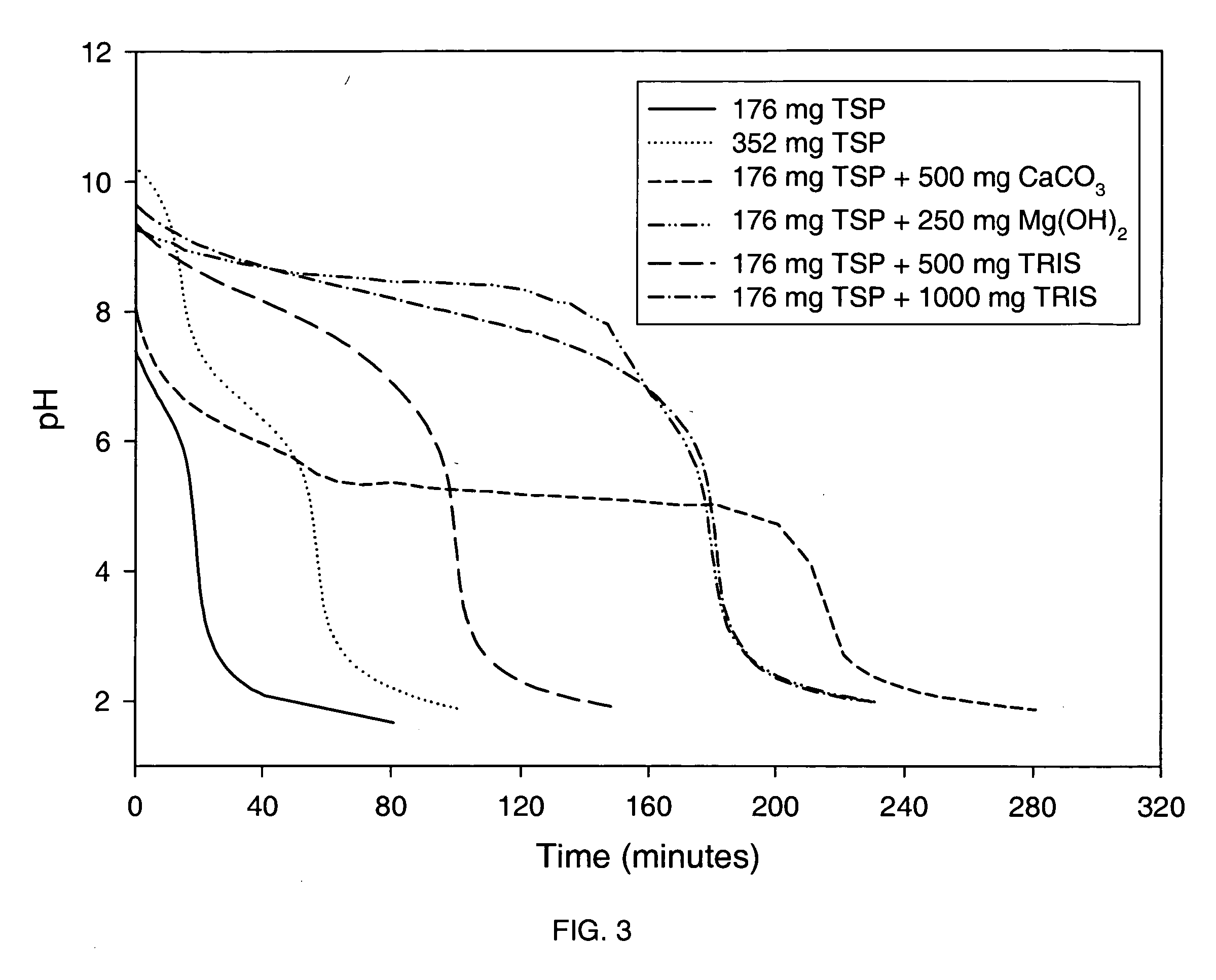
Azithromycin dosage forms with reduced side effects
ActiveUS6984403B2Reduce gastrointestinal side effectsAntibacterial agentsPowder deliveryOral suspensionsGLYCERYL MONOBEHENATE
The present invention is related to an oral dosage form comprising an effective amount of an alkalizing agent and an azithromycin multiparticulate wherein said multiparticulate comprises azithromycin, a glyceride which comprises glyceryl monobehenate, glyceryl dibehenate, glyceryl tribehenate, or a mixture thereof and a poloxamer. Typically, the oral dosage form includes any suitable oral dosing means such as a powder for oral suspension, a unit dose packet or sachet, a tablet or a capsule.
Owner:PFIZER INC
Compounds and methods for lowering the abuse potential and extending the duration of action of a drug
InactiveUS20070203165A1Low affinityLow abuse potentialBiocideNervous disorderNasal cavityEster prodrug
Owner:CONTROLLED CHEM INC
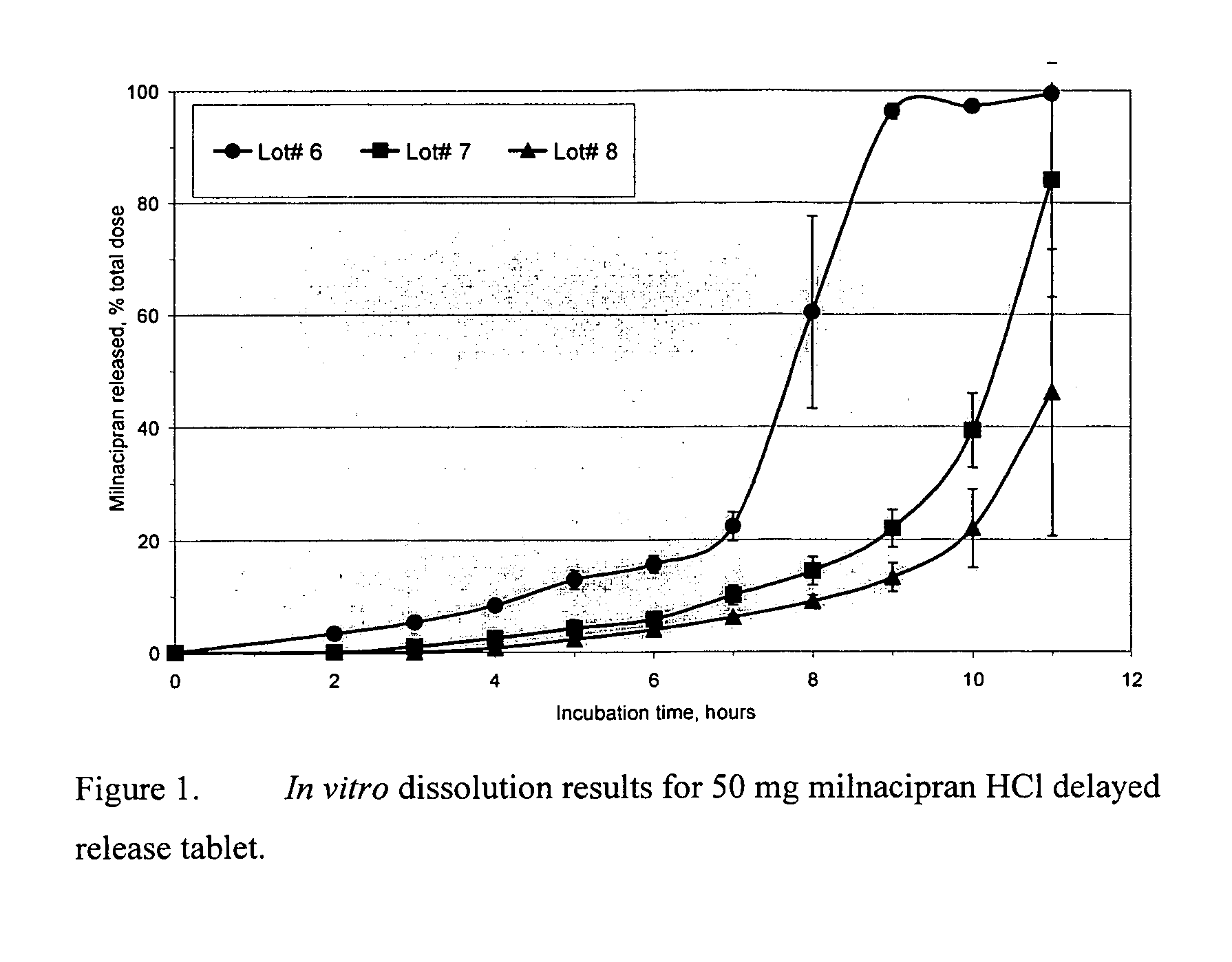
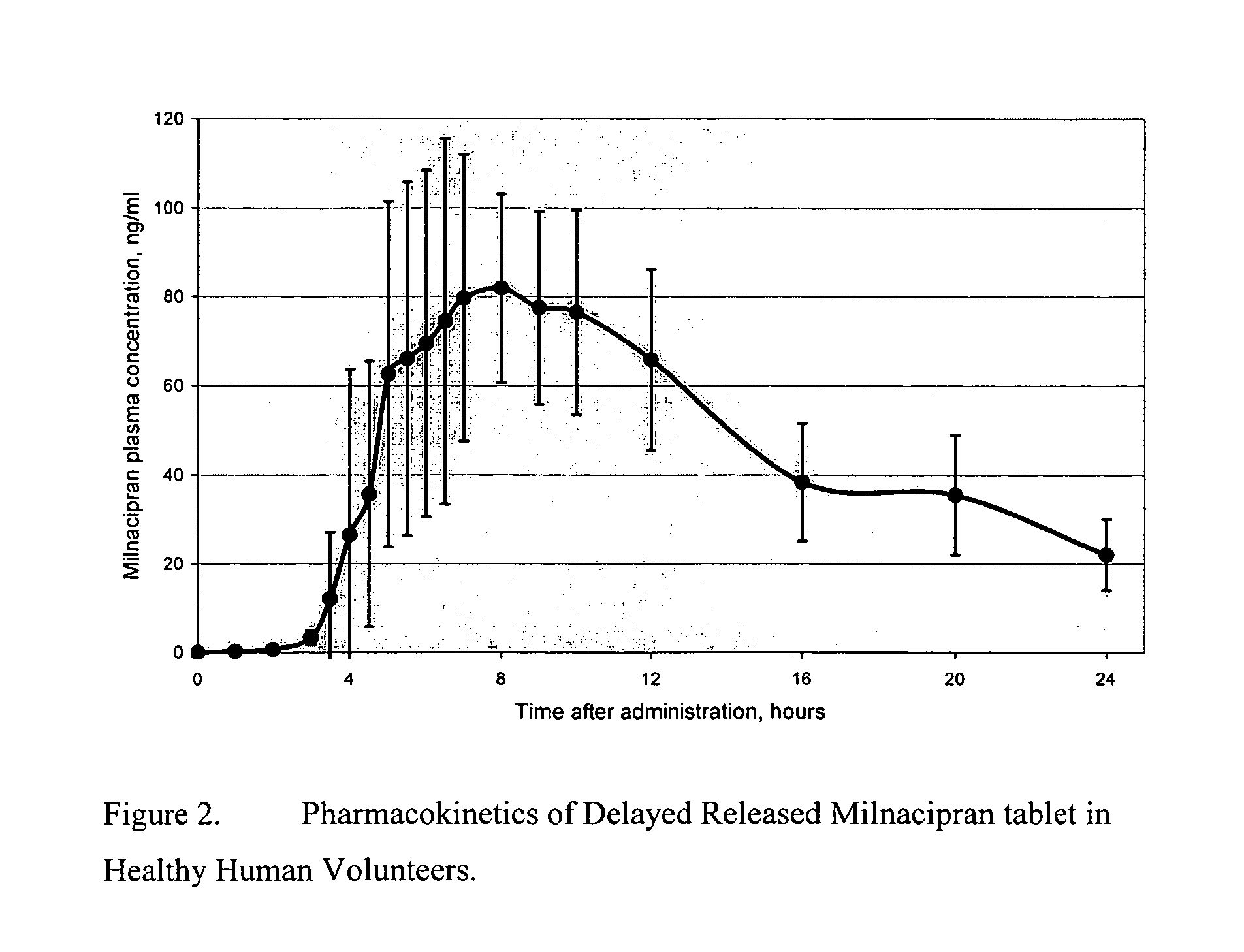
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
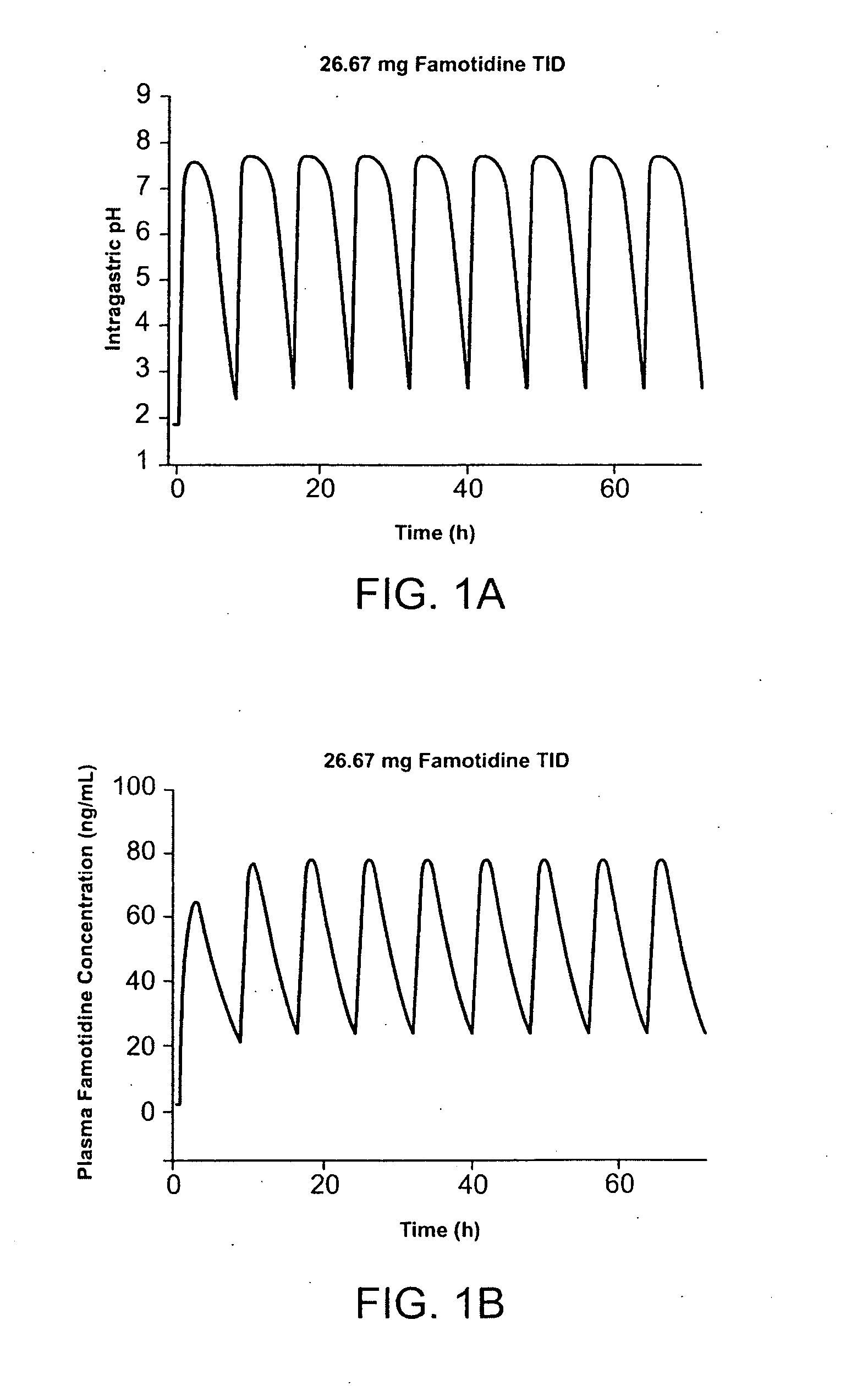
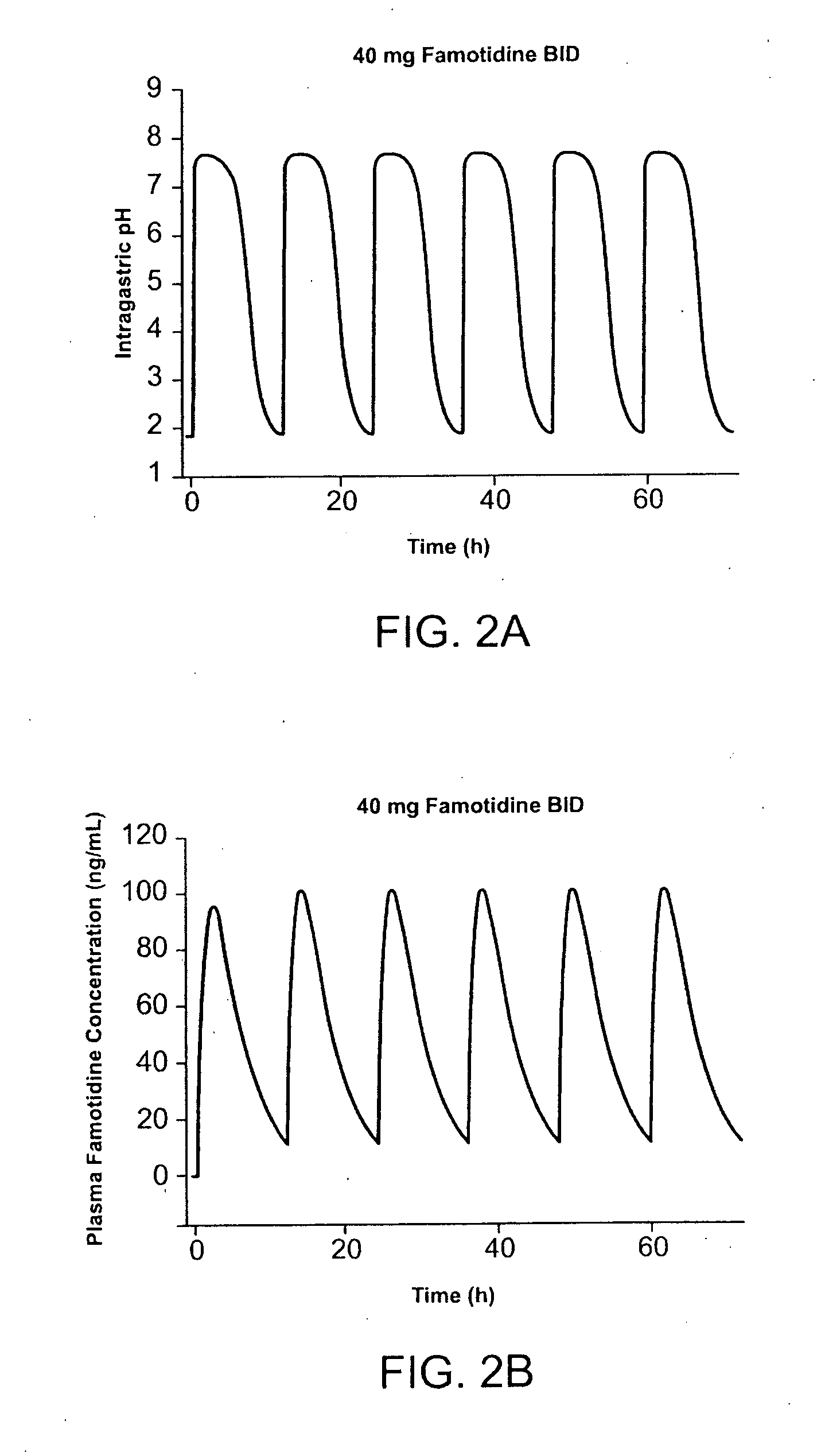
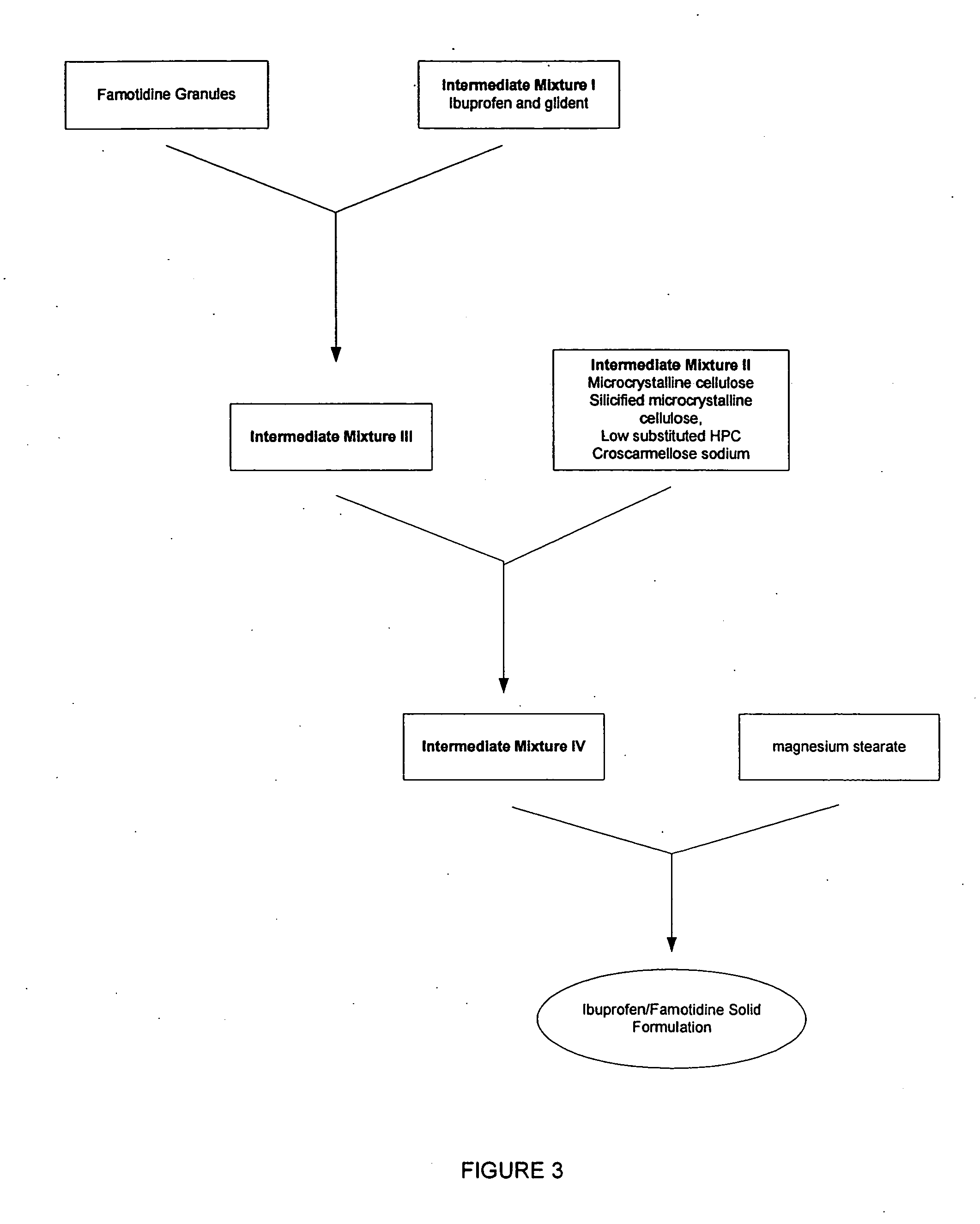
Unit dose form with ibuprofen-famotidine admixture
InactiveUS20070043096A1Reduce gastrointestinal side effectReduce gastrointestinal side effectsBiocideAntipyreticDosage formIbuprofen
An oral dosage form for administration of ibuprofen to a subject in need of ibuprofen treatment is provided, in which an oral dosage form comprising a therapeutically effective amount of ibuprofen and a therapeutically effective amount of famotidine, in admixture, in amounts suitable for three times per day administration.
Owner:HORIZON MEDICINES LLC
Pharmaceutical formulations containing a non-steroidal antinflammatory drug and an antiulcerative drug
InactiveUS20070237820A1Reduce gastrointestinal side effectsReduce riskSalicyclic acid active ingredientsGranular deliveryAnti-ulcer AgentPharmaceutical formulation
Disclosed is a pharmaceutical dosage form including a therapeutically effective amount of an NSAID and an antiulcerative agent.
Owner:ANDRX PHARMA INC
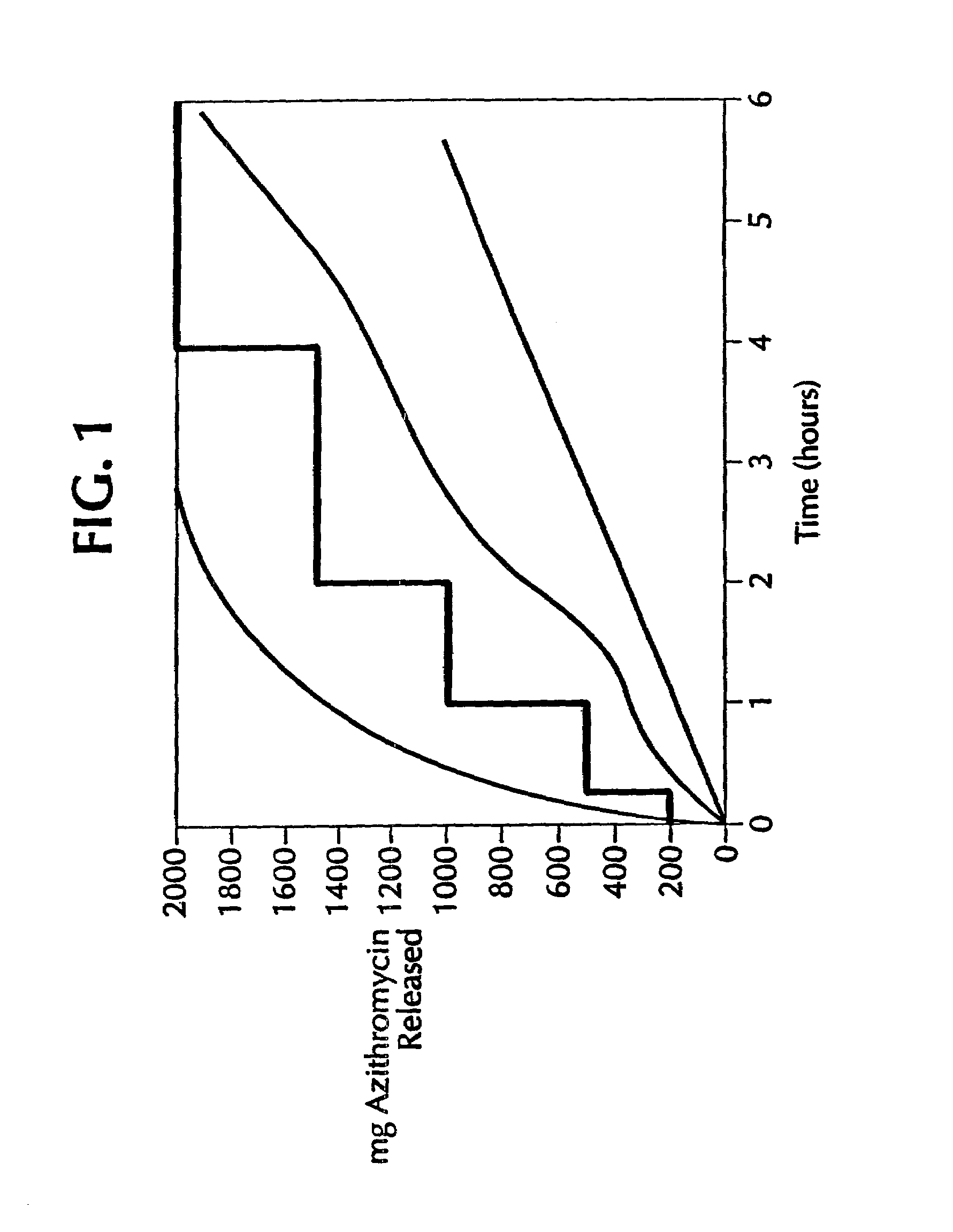
Controlled-release dosage forms of azithromycin
InactiveUS7108865B2Reduce incidenceReduce severityAntibacterial agentsBiocideAzithromycinSide effect
A controlled-release dosage form of azithromycin having an improved side effect profile; a process for preparing the dosage form; and a method of treating a microbial infection, comprising administering azithromycin in such a controlled-release dosage form to a mammal, including a human patient, in need of such treatment.
Owner:PFIZER INC
Orlistat compositions
InactiveUS20060269510A1Reduce gastrointestinal side effectsEliminate side effectsBiocideMetabolism disorderLipase inhibitorsObesity
Owner:BARBIER PIERRE +2
Methods and compositions for treatment of nicotine dependence and dementias
InactiveUS20050277626A1Easy to useIncrease heightBiocideNervous disorderTricyclic antidepressantTricyclic anti-depressants
In accordance with the present invention, it has been unexpectedly found that the administration of an acetylcholinesterase inhibitor in combination with a tricyclic antidepressant having anti-muscarinic properties provides a highly effective and well tolerated treatment for nicotine dependence and dementias, such as Alzheimer's disease (AD). In one aspect, it is effective in the treatment of nicotine dependence, as well as in the treatment and mitigation of nicotine withdrawal symptoms and in the effectuation of smoking cessation. In another aspect, it is effect in the treatment and mitigation of dementias such as Alzheimer's disease.
Owner:NEUROCURE
Enhanced compliance antiviral medicaments and methods of manufacture and use
InactiveUS20050281872A1Reduce gastrointestinal side effectsReducing gastrointestinal bleeding and the resultant patient discomfortBiocideCarbohydrate active ingredientsSide effectPatient compliance
Reduced gastrointestinal side effect medicaments for treating viral infection in a patient suffering therefrom are provided comprising at least 500 mg of antiviral compound in an oral dosage form that can be administered in effective total daily dosages of antiviral compound ranging from 1000 mg to 2000 mg. The reduced gastrointestinal side effect medicaments enhance patient compliance with long term, multi-dose treatment regimens by reducing physiological and psychological side effects that can cause reductions or discontinuations of antiviral therapy. Exemplary antiviral compounds include nucleoside analogues such as ribavirin, levovirin, and viramindine which are effective when combined with interferon to treat acute or chronic viral infections including hepatitis, and particularly hepatitis C. Associated methods for the production and use of the medicaments also are provided.
Owner:AXIUM HEALTHCARE PHARMACY
Azithromycin dosage forms with reduced side effects
ActiveUS20050123627A1Reduce gastrointestinal side effectsAntibacterial agentsBiocideOral suspensionsGLYCERYL MONOBEHENATE
An oral dosage form comprising azithromycin and an effective amount of an alkalizing agent. Preferably, said oral dosage form comprises an effective amount of an alkalizing agent and an azithromycin multiparticulate wherein said multiparticulate comprises azithromycin, a mixture of glyceryl monobehenate, glyceryl dibehenate and glyceryl tribehenate, and a poloxamer. Typically, the oral dosage form includes any suitable oral dosing means such as a powder for oral suspension, a unit dose packet or sachet, a tablet or a capsule. Additionally disclosed is an oral suspension comprising azithromycin, an effective amount of an alkalizing agent and a vehicle. Preferably, the azithromycin is in multiparticulate form wherein said multiparticulate comprises azithromycin, a mixture of glyceryl monobehenate, glyceryl dibehenate and glyceryl tribehenate, and a poloxamer. Also disclosed is a method for reducing gastrointestinal side effects, associated with administering azithromycin to a mammal, comprising contiguously administering azithromycin and an effective amount of alkalizing agent to said mammal wherein the frequency of gastrointestinal side effects is lower than that experienced by administering an equal dose of azithromycin without said alkalizing agent. Further disclosed is a method of treating a bacterial or protozoal infection in a mammal in need thereof comprising contiguously administering to said mammal a single dose of an oral dosage form wherein said oral dosage form comprises azithromycin and an effective amount of an alkalizing agent. Additionally disclosed are azithromycin multiparticulates comprising azithromycin, a surfactant; and a pharmaceutically acceptable carrier.
Owner:PFIZER INC
Venlafaxine hydrochloride controlled-release pellet and preparation method thereof
ActiveCN102935071AReduce viscosityOvercome coating electrostatic problemsOrganic active ingredientsNervous disorderSpray coatingSolvent
The invention provides a venlafaxine hydrochloride controlled-release pellet and a preparation method thereof. The venlafaxine hydrochloride controlled-release pellet comprises a blank pellet core, a venlafaxine hydrochloride-containing layer and a controlled-release film, wherein the venlafaxine hydrochloride-containing layer comprises active components including venlafaxine hydrochloride and talcum powder which are in a weight ratio of 12:1-8:1, and the weight ratio of the venlafaxine hydrochloride to the blank pellet core is 4:10-8:10. The venlafaxine hydrochloride controlled-release pellet is prepared by adopting a fluidized bed bottom-spraying venlafaxine hydrochloride applying mode; by using 75 percent alcohol as a solvent, the viscosity of the venlafaxine hydrochloride in a water solution is remarkably lowered, the venlafaxine hydrochloride applying speed is increased; and the prepared venlafaxine hydrochloride containing pellet adopts a bottom-spraying coating process, thus the problem of static electricity generated because of the adoption of the pure organic solvent is overcome; the application process and the wrapping process can be continuously operated in the same device, thus the production efficiency is increased and the production cost is lowered; and no toxic organic solvent is adopted in the whole process, thus the venlafaxine hydrochloride controlled-release pellet is environmental-friendly and is good in stability, and almost no broken pellets or dust is produced.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Sustained release preparation of roxithromycin
InactiveCN1415305AGood curative effectGreat tasteAntibacterial agentsOrganic active ingredientsRoxithromycinAdhesive
A showly-releasing roxithromycin contains roxithromycin (30-80 wt.%), slow-releasing assistant (hydroxypropylmethyl cellulose, etc) (10-40 wt.%), and others (hole-forming agent, adhesive, lubricant, etc). Its advantages are long active period (24 hrs.), and low by-effect.
Owner:SHENYANG PHARMA UNIVERSITY
Novel tryptamine derivative and preparation method and application thereof
PendingCN111116449AStrong inhibitory activityHas a neuroprotective effectOrganic active ingredientsNervous disorderInflammatory factorsSide effect
The invention discloses a novel tryptamine derivative. The novel tryptamine derivative is characterized in that the chemical structural formula of the novel tryptamine derivative is as shown in the specification. According to the invention, an intracerebral tryptamine hormone fragment with an anti-neuroinflammation effect and a non-steroidal anti-inflammatory drug salicylic acid and derivatives thereof are subjected to molecular combination; on one hand, COX effect can be inhibited; and on the other hand, the generation of iNOS and inflammatory factors and the activation of immune cells can beinhibited, and the occurrence and development of inflammations are jointly inhibited through multiple mechanisms. Meanwhile, carboxylic acid groups of salicylic acid non-steroidal anti-inflammatory drugs can be removed, so gastrointestinal side effects caused by acidity are reduced. On such a basis, a series of compounds are designed and synthesized; and enzyme inhibition tests, various immune cell tests and animal in-vivo experimental studies prove that the synthesized compounds have good anti-inflammatory and analgesic activity.
Owner:LANZHOU UNIVERSITY
Prenatal multivitamin/multimineral supplement
InactiveUS7994217B2Reduce gastrointestinal side effectsProblem can be addressedHeavy metal active ingredientsBiocideIron saltsPhysiology
Multivitamin / Multimineral supplements are provided for supplementing iron and desirable nutrients in the diet of mammals. The supplements include an iron-amino acid chelate, a form of iron more bioavailable than traditional iron salts. The supplements may further include a pharmaceutically accepted salt form of iron as well as other nutritional vitamins and minerals. The supplements are useful for providing iron for pregnant and lactating females as well as for persons suffering from anemia.
Owner:XANODYNE PHARMACEUTICALS INC
Wingedtooth laggera herb naphtha extracts, method for preparing wingedtooth laggera herb naphtha extracts, medicinal compositions and application of wingedtooth laggera herb naphtha extracts or medicinal compositions
ActiveCN102100719AThe basic requirements of modernization meetHigh purityAerosol deliverySuppositories deliveryNaphthaCurative effect
The invention provides wingedtooth laggera herb naphtha extracts with a definite curative effect and clear structure, a method for preparing the wingedtooth laggera herb naphtha extracts, medicinal compositions for treating acute and chronic pharyngitis and application of the wingedtooth laggera herb naphtha extracts or the medicinal compositions. When prepared into a spraying agent, the extracts have the prominent advantage of treating respiratory diseases, and active ingredients can directly reach the affected part, so that the first pass effect is avoided, the bioavailability of the extracts is improved, the dosage of the extracts is reduced, and the adverse reactions caused by the extracts are reduced or eliminated.
Owner:YUNNAN PANLONG YUNHAI PHARMA
Tegafur and gimeracil compound injecta
ActiveCN102309491AReduce gastrointestinal side effectsQuick effectOrganic active ingredientsPharmaceutical delivery mechanismChemistryOTERACIL POTASSIUM
The invention discloses tegafur and gimeracil compound injecta, comprising tegafur, gimeracil, one or several kinds of auxiliaries suitable for injecta, and the balance of water for injection, wherein the tegafur and the gimeracil serve as active constituents, and the tegafur and gimeracil compound injecta is characterized in that the molar ratio of tegafur to gimeracil to oteracil potassium is 1:(0.2-0.4):1.
Owner:TIANJIN JINYAO GRP
Treatment and Prevention of Gastrointestinal Syndrome
PendingUS20140255518A1Maintain genomic integrityReduce gastrointestinal damageHormone peptidesBiocidePhysiologyChemotherapy
Compositions comprising a guanylyl cyclase C agonist in an amount effective to protect intestinal tissue against radiation or chemotherapy and methods of using such compositions to prevent GI syndrome in cancer patient undergoing radiation or chemotherapy and in individuals exposed to or susceptible to exposure to radiation are disclosed.
Owner:THOMAS JEFFERSON UNIV HOSPITALS
Cefepime hydrochloride medicine composition, powder-injection thereof and preparation method thereof
InactiveCN102743390BImproves pH stabilityUniform product qualityAntibacterial agentsPowder deliveryCefepime hydrochlorideSide effect
The invention relates to a cefepime hydrochloride medicine composition, a powder-injection thereof and a preparation method thereof. The medicine composition comprises the following ingredients: cefepime hydrochloride, L-arginine and hydroxypropyl-beta-cyclodextrin, wherein the weight of the L-arginine is 40% of that of the cefepime hydrochloride, and the weight of the hydroxypropyl-beta-cyclodextrin is 10% of that of the cefepime hydrochloride. The medicine composition is good in mixing uniformity, has small pH value change in subpackaging and transportation processes, is small in gastrointestinal tract side effect, and greatly improves the stability and safety of the medicine.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Combination of a nicotinic receptor partial agonist and of an acetylcholinesterase inhibitor, pharmaceutical composition containing same and use thereof in the treatment of cognitive disorders
InactiveCN102046164AWell toleratedReduce gastrointestinal side effectsNervous disorderAmine active ingredientsCognitive disorderAcetylcholinesterase inhibitor
he present invention relates to the combination of an alpha-7 nicotinic receptor partial agonist and of an acetylcholinesterase inhibitor. The invention also relates to a pharmaceutical composition comprising the combination according to the invention and to the use thereof in the treatment of cognitive disorders.
Owner:SANOFI AVENTIS SA
Method of reducing CNS and gastrointestinal side affects associated with long-term dextromethorphan/low-dose quinidine combination therapy
InactiveUS20110212987A1Reduce gastrointestinal side effectsRelieve nauseaBiocideNervous disorderSide effectQuinidine
Pharmaceutical compositions and methods for treating neurological disorders by administering same are provided. The compositions comprise dextromethorphan in combination with quinidine. This invention also provides methods of reducing CNS and gastrointestinal side effects associated with a long term, dextromethorphan / low-dose quinidine combination therapy.
Owner:AVANIR PHARMA
Orlistat compositions
InactiveUS20050136030A1Reduce gastrointestinal side effectSide-effect be reduceBiocideMetabolism disorderIntestinal Lipase InhibitorGastroenterology
Owner:BARBIER PIERRE +2
Tegafur, gimeracil and oteracil potassium compound injecta
InactiveCN102309492AReduce gastrointestinal side effectsQuick effectPharmaceutical delivery mechanismAntineoplastic agentsOTERACIL POTASSIUMChemistry
The invention discloses tegafur, gimeracil and oteracil potassium compound injecta, comprising tegafur, gimeracil, oteracil potassium, one or several kinds of auxiliaries suitable for injecta, and the balance of water for injection, wherein the tegafur, the gimeracil and the oteracil potassium serve as active constituents, and the tegafur, gimeracil and oteracil potassium compound injecta is characterized in that the molar ratio of tegafur to gimeracil to oteracil potassium is 1:(0.2-0.4):1.
Owner:TIANJIN JINYAO GRP
Compound aspirin tading kind medicinal preparation and its application
InactiveCN1651085AImprove stabilityInhibitory activityOrganic active ingredientsMetabolism disorderAspirinStatine
Owner:FUKANGREN BIO PHARMA
Pharmaceutical bead formulations comprising dimethyl fumarate
ActiveUS11197842B2Reduce gastrointestinal side effectsMaximize absorptionOrganic active ingredientsNervous disorderCombinatorial chemistryPharmaceutical Substances
Owner:BIOGEN MA INC
Biguanide derivative for preventing and treating infarction diseases and application thereof
ActiveCN109928897AReduce cerebral infarct size and cardiac infarct sizeCerebral infarction and myocardial infarction improvedOrganic chemistryBlood disorderBiguanideSide effect
The invention provides a biguanide derivative for preventing and treating infarction diseases and application of the biguanide derivative in preparation of a medicine for preventing and treating the infarction diseases. The biguanide derivative has an antiplatelet effect, has an obvious inhibition effect on formation of pulmonary embolism, arterial thrombosis and venous thrombosis, can effectivelyreduce cerebral infarction area and cardiac infarction area, and has an obvious improvement effect on cerebral infarction and myocardial infarction. The effect of the derivative is obviously better than that of a positive control medicine, aspirin. The biguanide derivative can also reduce gastrointestinal side effects and bleeding risks commonly existing in existing clinical antithrombotic drugs.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
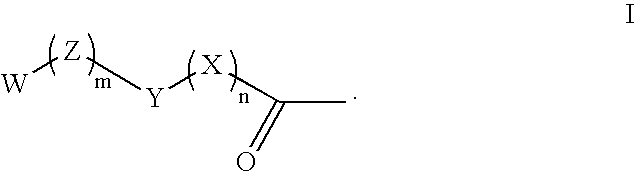
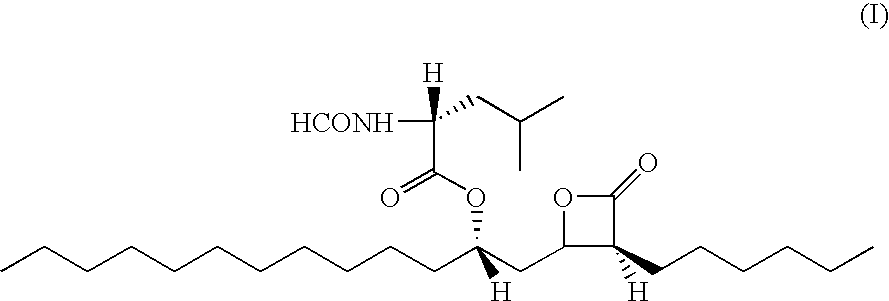
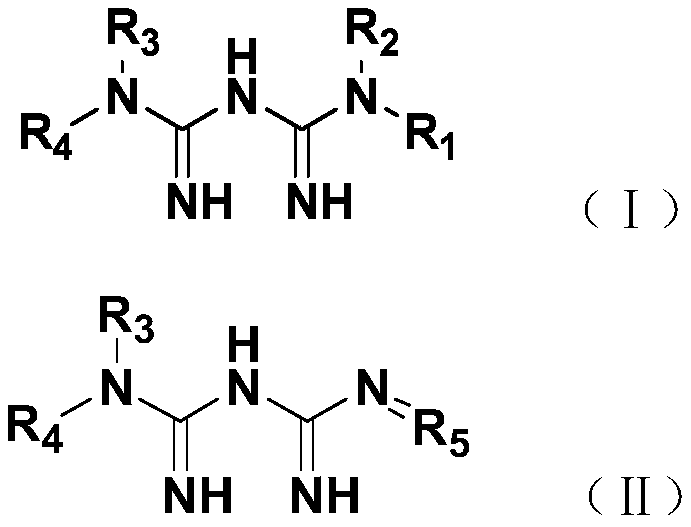
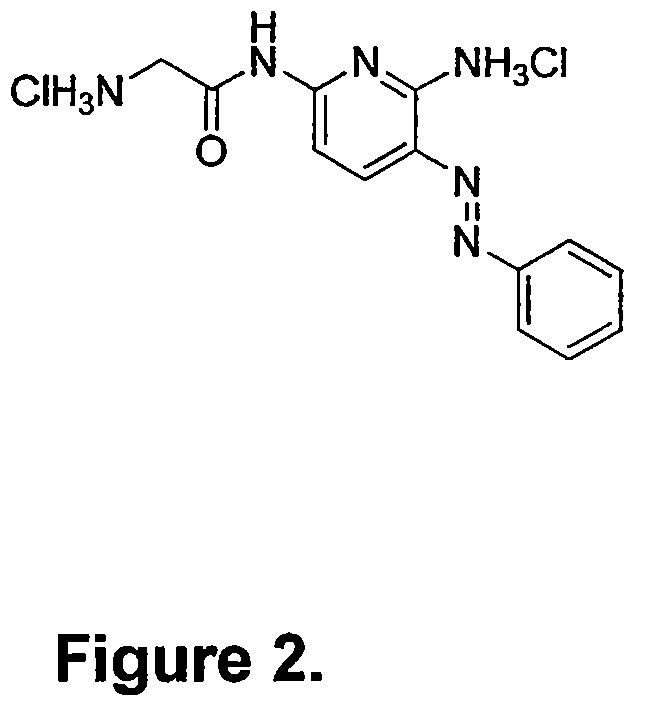
Phenazopyridine compounds
The present invention is directed to substituted phenazopyridines represented by Formula I. The present invention also relates to the discovery that compounds of Formula I have increased bioavailability as compared to unconjugated phenazopyridine.
Owner:PINNACLE PHARMA INC
COX-2 selective carprofen for treating pain and inflammation in dogs
InactiveCN1255059AReduce gastrointestinal side effectsOrganic chemistryAntipyreticDiseaseM-aminophenol
Treating or preventing inflammatory processes and diseases in dogs associated with the activity of inducible cyclo-oxygenase-2 (COX-2), while at the same time reducing or eliminating undesirable side effects associated with simultaneous inhibition of the activity of constitutive cyclo-oxygenase-1 (COX-1) by selectively inhibiting COX-2 activity with reference to COX-1 activity, wherein the selectivity ratio or COX-2 : COX-1 activity inhibition is at least 3:1 based on ex vivo inhibition levels measured in whole blood; the inhibitor is a member selected from the group of anti-inflammatory compounds consisting essentially of salicylic acid derivatives, p-aminophenol derivatives, indole and indene acetic acids, heteroaryl acetic acids, arylpropionic acids, anthranilic acids, enolic acids, and alkanones; the inhibitor in particular is comprised of (+)(S)-enantiomer of 6-chloro-alpha-methyl-9H-carbazole-2-acetic acid.
Owner:PFIZER INC
Rivastigmine-containing sustained-release pharmaceutical composition
ActiveUS20180125785A1Reduce gastrointestinal side effectsGood treatment effectNervous disorderPharmaceutical non-active ingredientsSide effectBlood concentration
The present invention relates to a rivastigmine-containing sustained-release pharmaceutical composition and, more specifically, to a rivastigmine-containing sustained-release pharmaceutical composition, which is a sustained-release preparation containing a pH-dependent delayed release phase, wherein, by controlling the release of the pharmaceutical composition to be minimized in the stomach at the initial stage of administration, the pharmaceutical composition can lower the maximum blood concentration (Cmax) compared with existing products while arriving at an effective blood concentration, thereby reducing side effects, and thereafter, maintaining the effective blood concentration through the sustained-release of main ingredients. As a result, the pharmaceutical composition according to the present invention exhibits the same effect as in the existing twice-a-day dosing through only the once-a-day dosing, and can increase the treatment efficiency of patients through the improvement in the convenience of administration of patients.
Owner:NAVIPHARM CO LTD
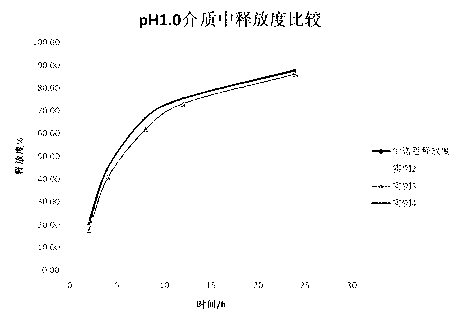
A kind of dimethyl fumarate enteric-coated pellets and preparation method thereof
ActiveCN104352441BReduce gastrointestinal side effectsAvoid sublimationOrganic active ingredientsPharmaceutical non-active ingredientsMedicineEnteric coated pellets
The invention relates to a dimethyl fumarate enteric-coated pellet and a preparation method thereof. The dimethyl fumarate enteric-coated pellets contain a pellet core and double-layer enteric coatings coated on the pellet core. The enteric-coated pellets are prepared through the following methods, including: preparing pellet cores by extrusion spheronization; and performing double-layer enteric-coating on the pellet cores to obtain enteric-coated pellets. The dimethyl fumarate enteric-coated pellets obtained by the invention have a simple preparation process, complete drug release and good reproducibility, and reduce the risk of in vitro dose dumping.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Benpipralin compound preparation for treating cough and preparation method of benpipralin compound preparation
InactiveCN114522163ALower metabolismIncrease blood concentrationOrganic active ingredientsDigestive systemEnzyme Inhibitor AgentPharmaceutical Aids
The invention discloses a benazolin compound preparation for treating cough and a preparation method of the benazolin compound preparation. The compound preparation comprises the following components: benpipralin and cimetidine in a ratio of (0.01-0.03): (0.1-0.3). The raw material medicines in corresponding parts are taken, proper auxiliary materials are added according to corresponding preparation methods of pharmaceutics, and the raw material medicines can be respectively prepared into dosage forms such as tablets, capsules, solutions, syrup, dry suspensions or granules. Benpipralin is a peripheral non-addictive cough relieving medicine and is mainly used for relieving cough caused by various reasons. The cimetidine is a histamine H2 receptor impedance agent, is a hepatodrug enzyme inhibitor, and can inhibit the activity of hepatodrug enzyme and slow down the metabolism of benpipralin, so that the blood concentration of benpipralin is improved, and the curative effect of benpipralin is enhanced; and the cimetidine alleviates the stimulation of the benpipralin to the stomach by inhibiting gastric acid secretion, and reduces the gastrointestinal tract side effect of the benpipralin.
Owner:徐娴娴
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com