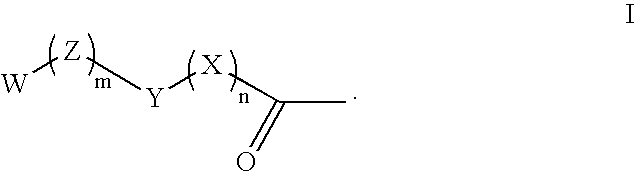
Compounds and methods for lowering the abuse potential and extending the duration of action of a drug
a technology of abuse potential and compound, which is applied in the field of compound and method for lowering the abuse potential and extending the duration of action of a drug, can solve the problems of serious abuse of oxycontin®, toxic levels of active drugs, and large abuse potential of these formulations, so as to reduce side effects, prolong the duration of action, and reduce the abuse potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of pentanedioic acid mono-(3-methoxy-14-hydroxy-6,7-didehydro-4,5α-epoxy-17-methylmorphinan-6-yl)ester (1-4, also designated compound 2)
Step A: Preparation of Oxycodone Free Base (1-1)
[0101] Oxycodone (Ig) was dissolved in water (5 mL) and mixed with 30 mL of a saturated sodium bicarbonate solution to produce the free base. The resulting suspension was extracted with three 70 mL portions of EtOAc. The combined EtOAc extract was washed with 30 mL of saturated sodium bicarbonate, 30 mL of brine and dried over magnesium sulfate. EtOAc was removed under reduced pressure from the resulting solution to yield 785 mg of oxycodone free base.
Step B: Preparation of Pentanedioic Acid Mono-Tert-Butyl Ester (1-2)
[0102] Potassium tert-butoxide (2.7 g, 24 mmol) was dissolved in 17 mL of anhydrous THF at rt. After 5 min glutaric anhydride (2.4 g, 21 mmol) was added and the resulting suspension stirred for 2 h at rt. The reaction mixture was then quenched with 20 mL of 1 M KHSO4, extr...
example 2
Preparation of phthalic acid mono-(3-methoxy-4,5α-epoxy-17-methylmorphinan-6-one-14-yl) ester (2-1, also designated compound 10)
[0105] A solution comprised of oxycodone free base, 1-1, (63 mg, 0.2 mmol), phthalic anhydride (1.185 g, 8.0 mmol) and DMAP (24 mg, 0.2 mmol) in 10 mL of pyridine was stirred in an oil bath at 50-55° C. for 24 h and concentrated under reduced pressure. The residue was subjected to silica gel flash chromatography with a 5%-20% methanol in dichloromethane gradient. The fraction containing 2-1 was collected and concentrated under reduced pressure. HPLC indicated that the fraction was 60% pure. The concentrated fraction was subjected to another silica gel flash chromatography using a gradient of 0-20% methanol in dichloromethane as eluent to yield a fraction containing 32 mg (35% yield) of 96% pure (HPLC) 2-1, which was further purified by HPLC. The 1H and 13C NMR spectra verified the structure of 2-1 as a hydrogen phthalate ester of the 14-hydroxyl group of o...
example 3
Preparation of 2-(benzyloxycarbonylamino)-pentanedioic acid 1-(3-methoxy-14-hydroxy-6,7-didehydro-4,5α-epoxy-17-methylmorphinan-6-yl) ester (3-2, also designated compound 1)
Step A: Preparation of 2-(benzyloxycarbonylamino)-pentanedioic acid 5-tert-butyl ester 1-(3-methoxy-14-hydroxy-6,7-didehydro-4,5α-epoxy-17-methylmorphinan-6-yl) ester (3-1)
[0106] A solution comprised of oxycodone free base, 1-1, (517 mg, 1.64 mmol), DIEA (1.5 mL, 8.6 mmol) in 9 mL of anhydrous acetonitrile was stirred at rt for 20 min and mixed with a solution containing DMAP (400 mg, 3.3 mmol), DCC (1.01 g, 4.1 mmol), and HOBt (440 mg, 3.3 mmol) in 6 mL of anhydrous acetonitrile. Cbz-L-Glu(OtBu)-OH (1.1 g, 3.3 mmol) was then added to the combined solutions. The mixture was stirred for 45 h at rt, precipitated DCU removed by filtration, and the solution concentrated under reduced pressure to give a dark-brown oil. HPLC analysis indicated that 39% of the oxycodone had been converted to 3-1. The brown oil contain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ri | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com