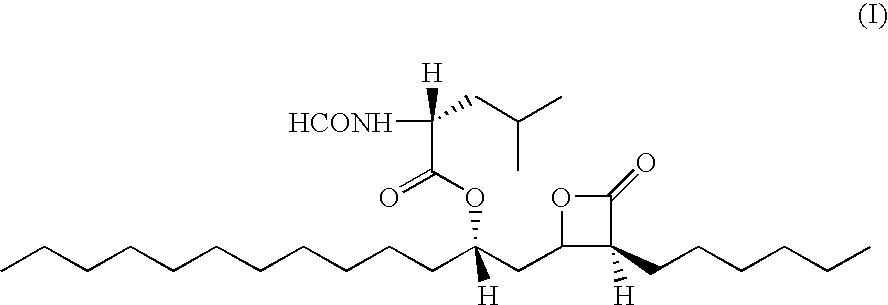
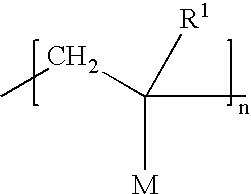
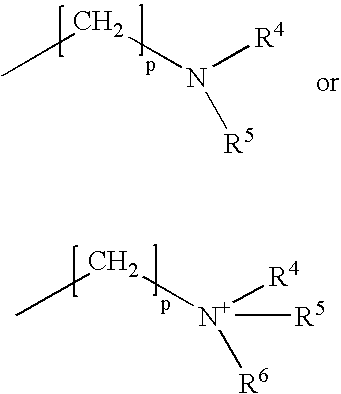
Orlistat compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Study A
[0067] Xenical was ingested t.i.d. by two middle aged healthy male volunteers on a normal average mixed diet. Both individuals frequently experienced one or more of the above mentioned unpleasant gastrointestinal side effects. After 4 weeks on Xenical they started to ingest in addition to Xenical b.i.d. cholestyramine containing sachets (4 g / meal) which were emptied into about 100 ml water, swirled and drunk during the meals. The side effects were immediately reduced in frequency and completely disappeared. After 2-4 weeks of combined intake together with Xenical, cholestyramine was discontinued. When treatment with Xenical alone was carried on the gastrointestinal adverse events reappeared.
example 2
Study B
[0068] The anti-GI side effect potential of bile salt sequestrants was extended further in short-term studies in human volunteers. To precipitate the tendency for side effects in this model three meals (lunch, dinner, breakfast) are ingested together with Xenical and 120 mg orlistat in 10 g butter, each. The model is based on the idea that GI side effects following orlistat ingestion are precipitated by the formation of free oil. Free oil is oil, which will coalesce in the dietary fat emulsion passing down the GI tract and separate from the stool matrix. In this model the amount of oil which is separated from the stool matrix is determined after the production of stools.
[0069] It was demonstrated in human volunteers that after co-administration of cholestyramine and colestipol, respectively, the formation of free oil was dramatically reduced (cholestyramine: 44% of the respective control experiment without addition of cholestyramine) or nearly completely suppressed (colesti...
example 3
Orlistat Pharmaceutical Compositions
[0070]
A)IngredientQuantity mg / Capsuleorlistat120.00microcrystalline cellulose (AVICEL PH-101)93.60sodium starch glycolate (PRIMOJEL)7.20sodium lauryl sulfate7.20polyvinylpyrrolidone (Povidone (K-30))12.00purified Water*—talc0.24Total240.24mg
*Removed during processing
Procedure:
[0071] Blend orlistat, microcrystalline cellulose, and sodium starch glycolate in a suitable mixer. Granulate with a solution of polyvinylpyrrolidone and sodium lauryl sulfate in purified water.
[0072] Pass the granulation through an extruder and pass the extrudate through a spheronizer to form pellets.
[0073] Dry the pellets at 30° C.
[0074] Add talc and mix.
[0075] Fill into hard gelatin capsules.
B)IngredientQuantity mg / Capsuleorlistat60microcrystalline cellulose46.8sodium starch glycolate3.6sodium lauryl sulfate3.6polyvinylpyrrolidone6.0purified water*—talc0.12Total120.12mg
*Removed during processing.
Procedure:
[0076] Blend orlistat, microcrystalline cellulose, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com