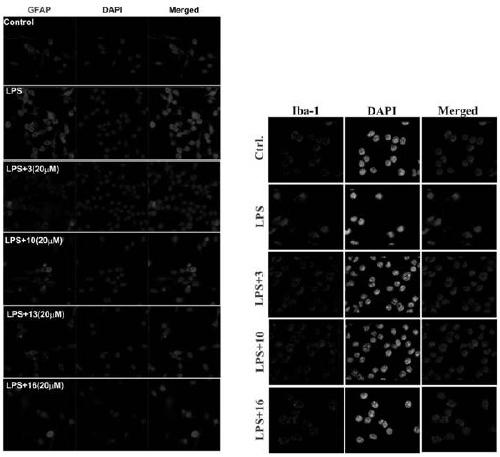
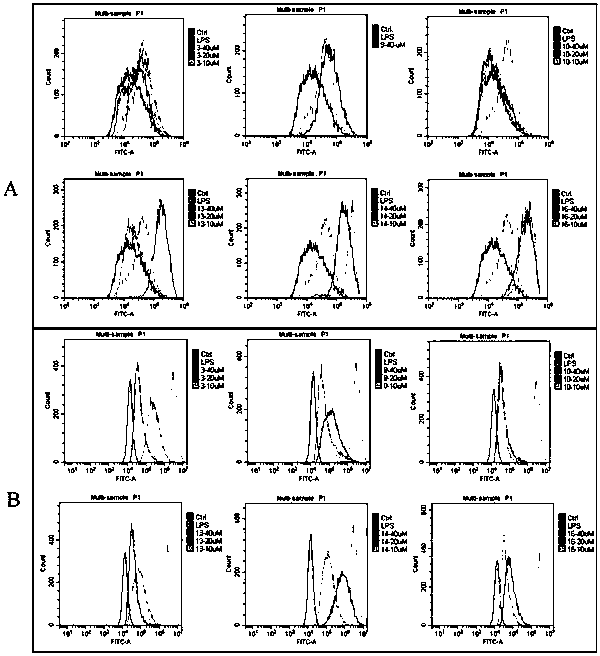
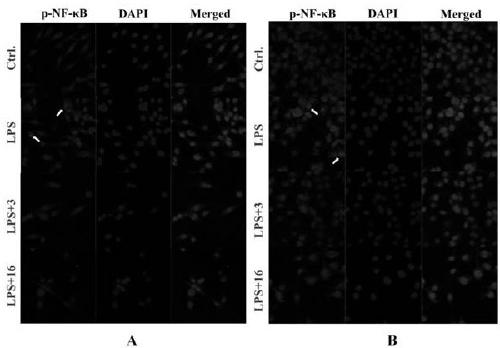
Novel tryptamine derivative and preparation method and application thereof
A derivative, tryptamine technology, applied in the field of medicine, can solve the problems of lack of anti-inflammatory activity research, in-depth research on anti-inflammatory structure-activity relationship, unclear curative effect and application, etc., to improve spatial memory and learning ability, pharmacokinetics, etc. The effect of optimal kinetic parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] N-(2-(1H-indol-3-yl)ethyl)-2-((3-chloro-2-methylphenyl)amino)benzamide (new tryptamine derivative 1): Add tryptamine ( Formula I, 0.60 mmol) (if formula I is hydrochloride, water and dichloromethane need to be added, the pH of the aqueous phase is adjusted to 11 for extraction, and dried), and 2.5 mL of solvent dichloromethane is added. Add EDCI condensation agent at room temperature, that is, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (0.60mmol), and simultaneously add catalyst HOBt (1-hydroxybenzotriazole) (0.50mmol) , base triethylamine (1.2mmol), and 2-((3-chloro-2-methylphenyl)amino)benzoic acid (formula II, 0.50mmol) were added and reacted at room temperature for 8h. After the completion of the reaction as monitored by TLC, it was filtered with suction, and the white solid was washed repeatedly with dichloromethane. The organic phase was spin-dried, and the organic phase was dried over anhydrous magnesium sulfate. Purification by silica gel colum...
Embodiment 2
[0045] N-(2-(1H-indol-3-yl)ethyl)-3-hydroxy-2-naphthamide (new tryptamine derivative 2):
[0046] With the method of embodiment 1, tryptamine is formula (I) (formula I), 3-hydroxy-2-naphthamide is (formula II) yield 75%. 1 H NMR(400MHz,DMSO)δ12.13(s,1H),10.87(s,1H), 9.19(t,J=5.4Hz,1H),8.51(s,1H),7.84(d,J=8.2Hz ,1H),7.74(d,J=8.3Hz,1H),7.62(d,J=7.8Hz,1H),7.50(t,J=7.5Hz,1H),7.35(dd,J=11.6,6.0Hz ,2H), 7.28(s,1H),7.23(s,1H),7.08(t,J=7.5Hz,1H),7.00(t,J=7.4Hz,1H),3.66(dd,J=13.3, 7.0Hz, 2H), 3.03(t, J=7.3Hz, 2H). 13 C NMR(101MHz,DMSO)δ168.18, 155.64,136.38,136.11,129.54,128.78,128.30,127.32,126.67,125.88,123.76, 122.88,121.09,118.65,118.40,111.70,111.53,110.83,40.19,25.04.
Embodiment 3
[0048] N-(2-(1H-indol-3-yl)ethyl)-2-hydroxy-4-methylbenzamide (new tryptamine derivative 3): with the method of Example 1, tryptamine is formula (I) (formula I), 2-hydroxy-4-methylbenzamide is (formula II) yield 80%. 1 H NMR (400MHz, DMSO) δ12.73(s, 1H), 10.83(s, 1H), 8.87(t, J=5.4Hz, 1H), 7.73(d, J=8.0Hz, 1H), 7.58(d ,J=7.8Hz,1H),7.35(d,J=8.1Hz,1H),7.18(d,J=1.6Hz,1H),7.07(t,J=7.5Hz,1H),6.99(t,J =7.4Hz,1H), 6.70(d,J=9.2Hz,2H),3.57(dd,J=13.5,7.0Hz,2H),2.97(t,J=7.4Hz,2H),2.27(s,3H ). 13 C NMR (101MHz, DMSO) δ169.20, 160.45, 144.13, 136.34, 127.43, 127.29, 122.77, 121.04, 119.61, 118.36, 118.33, 117.62, 112.53, 111.70, 111.48, 319.90, 2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com