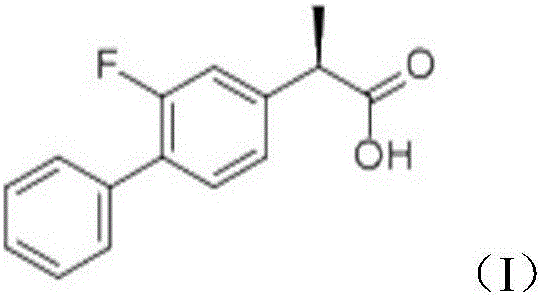
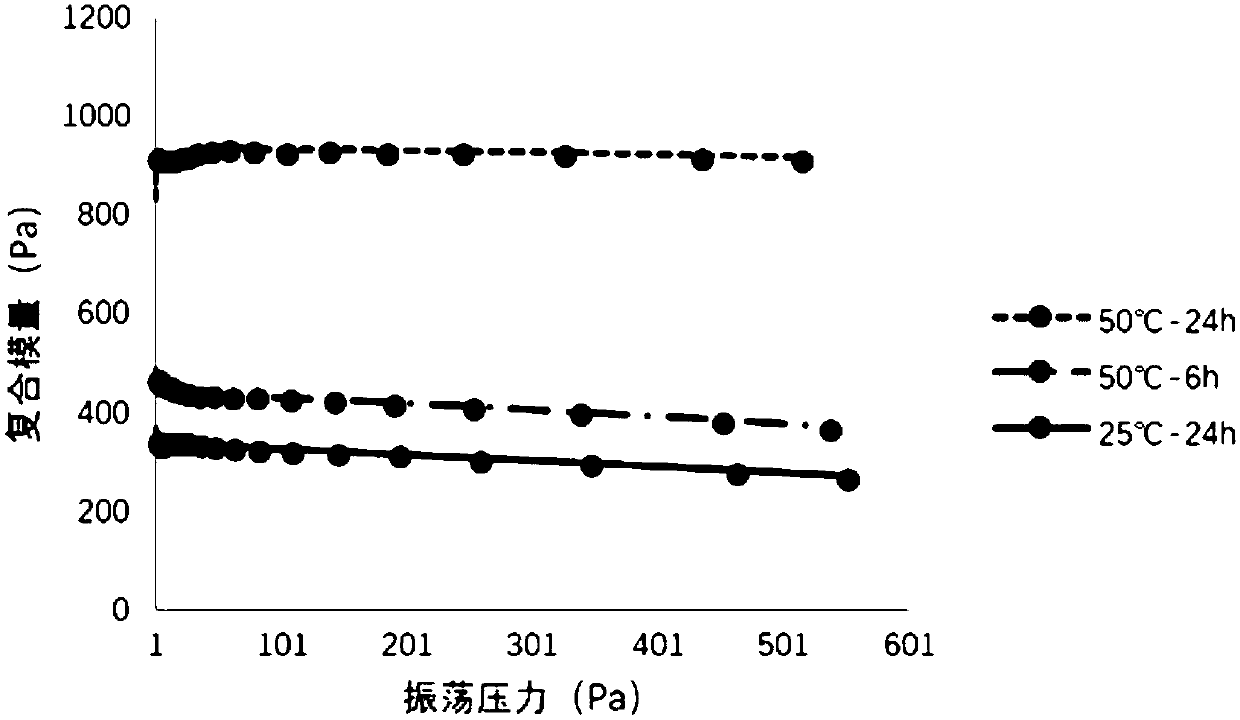
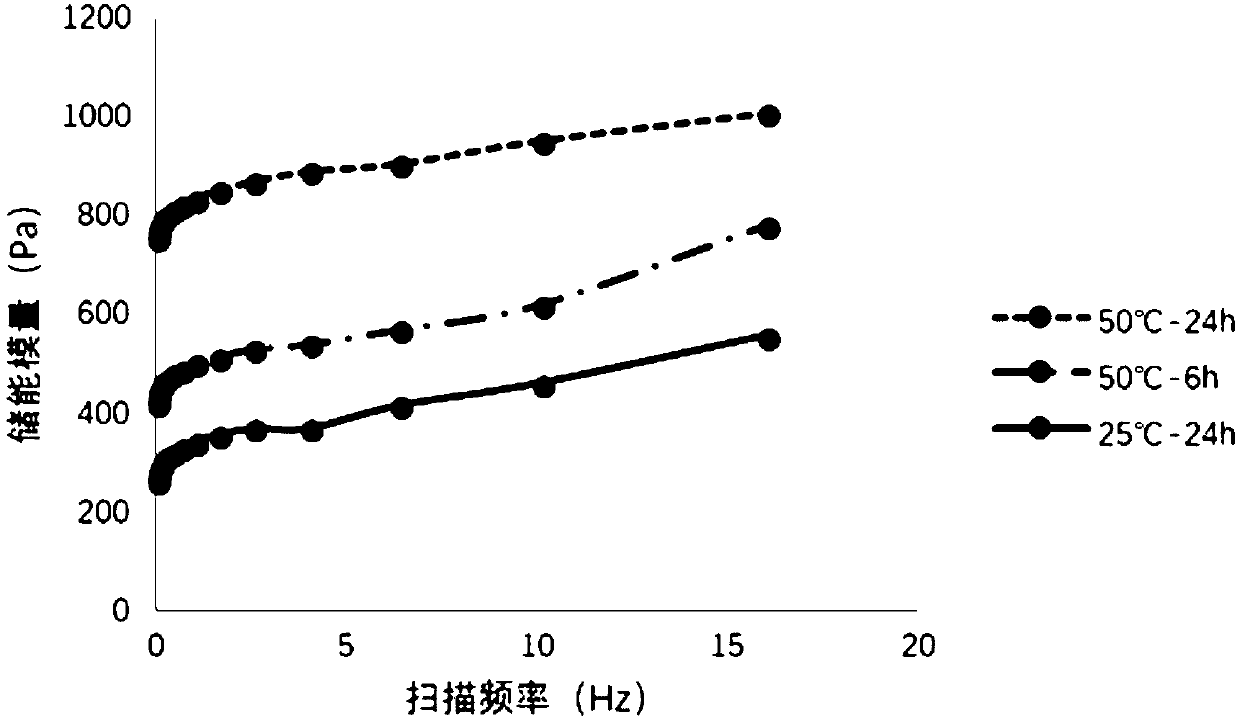
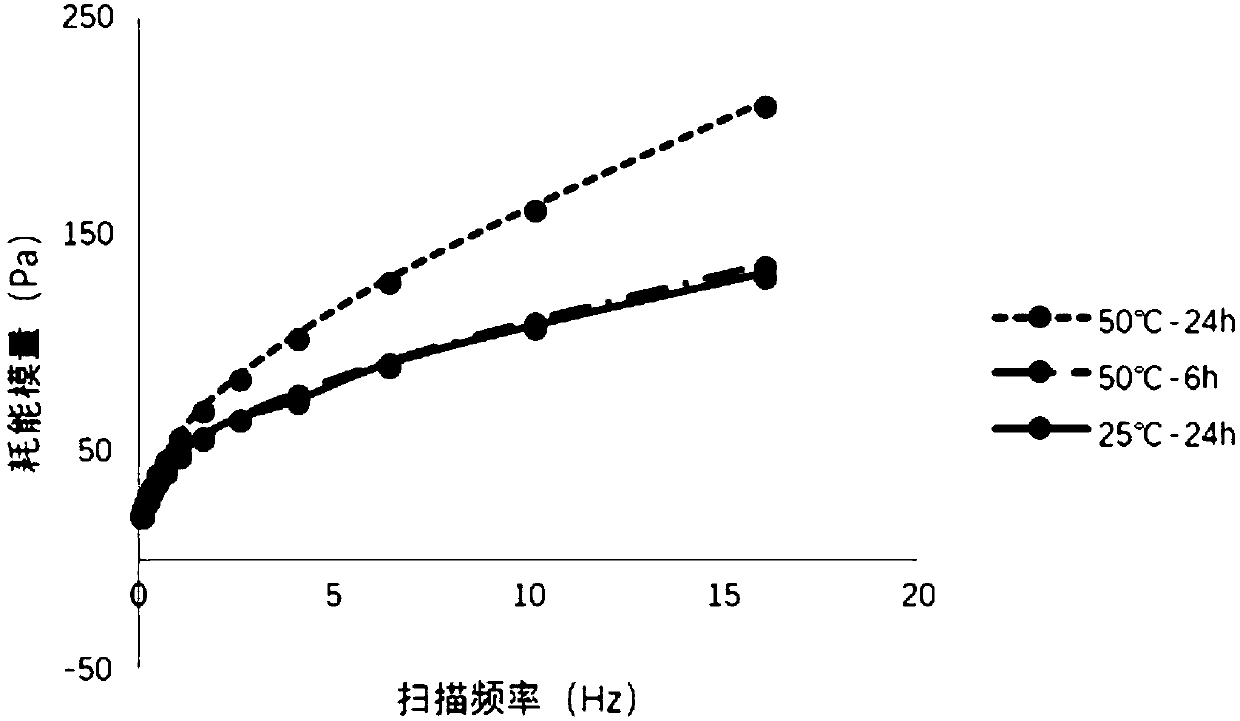
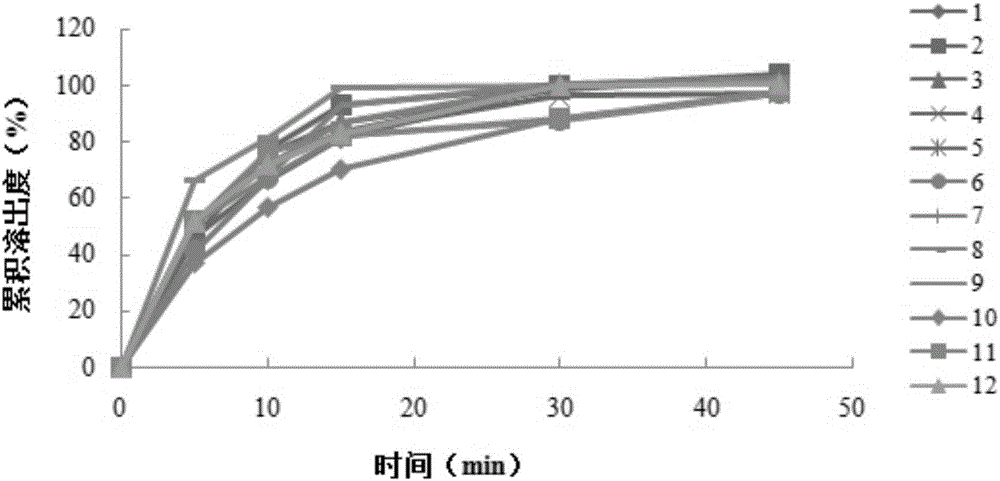
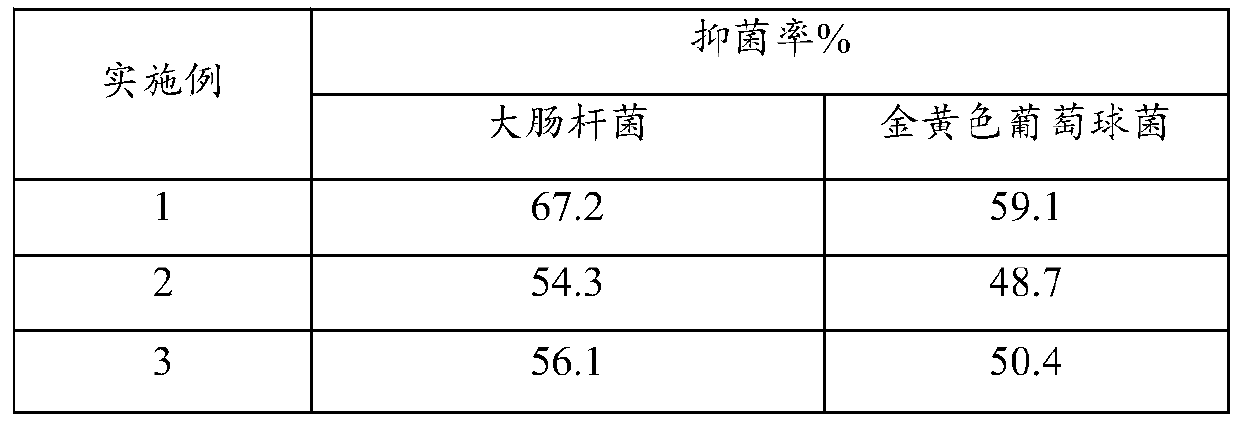
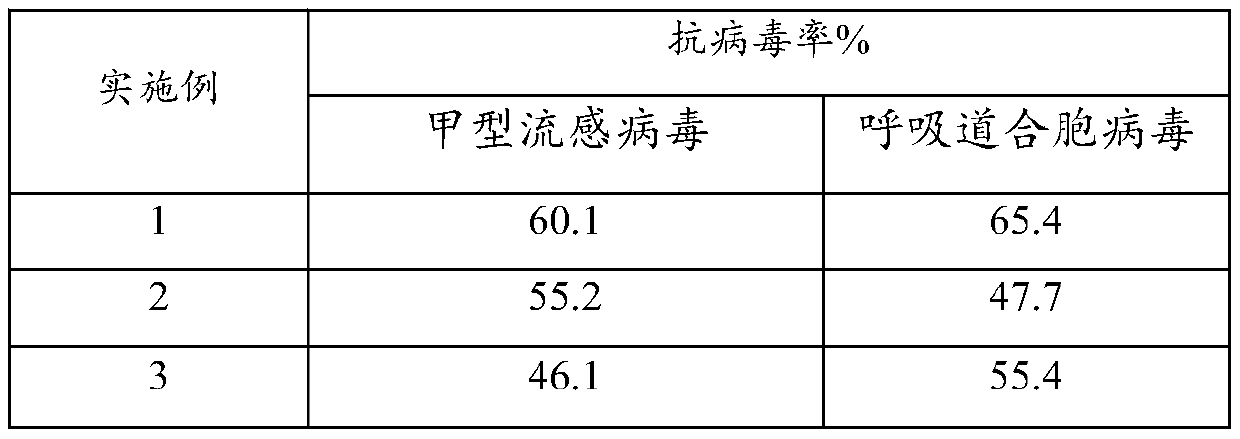
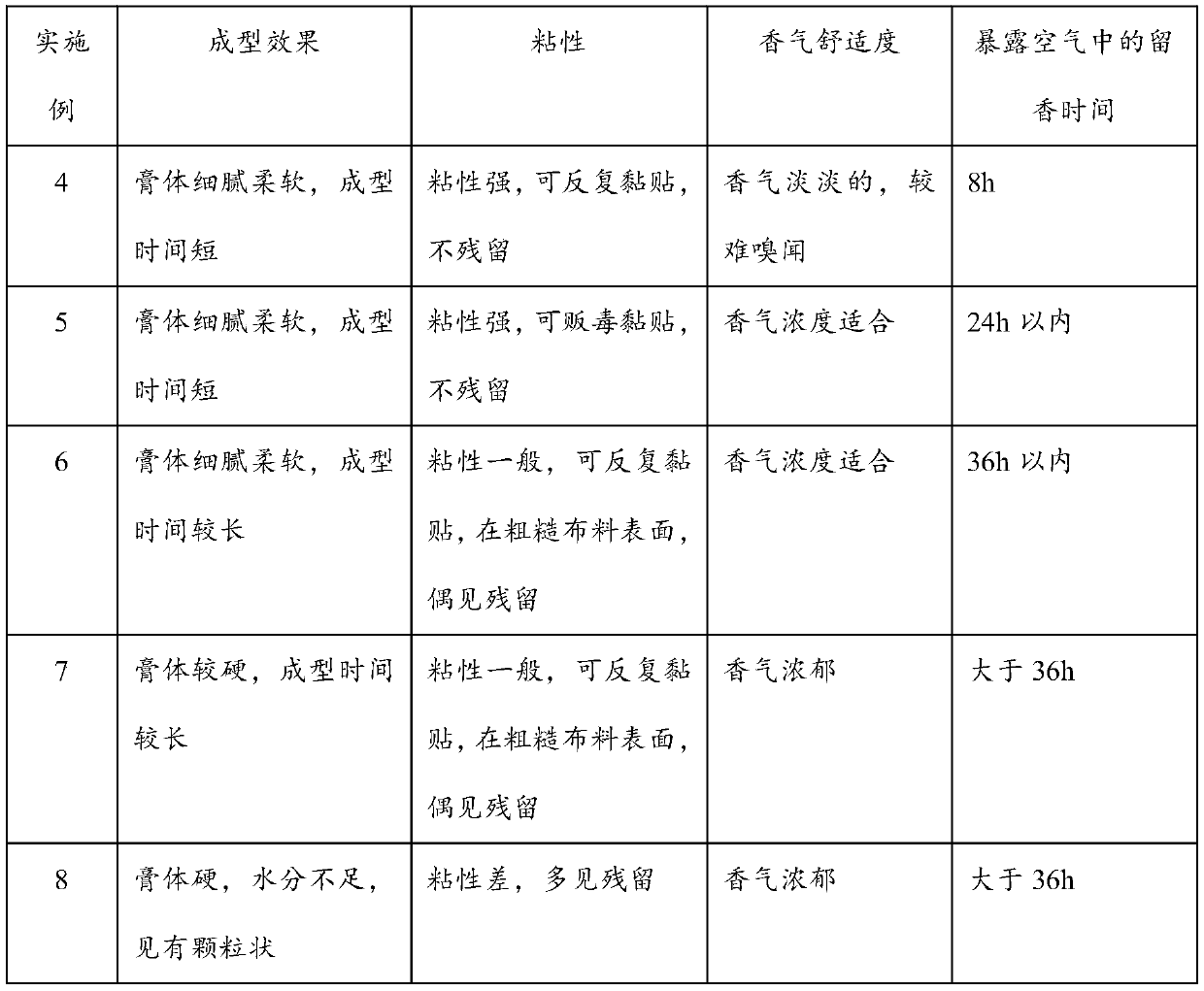
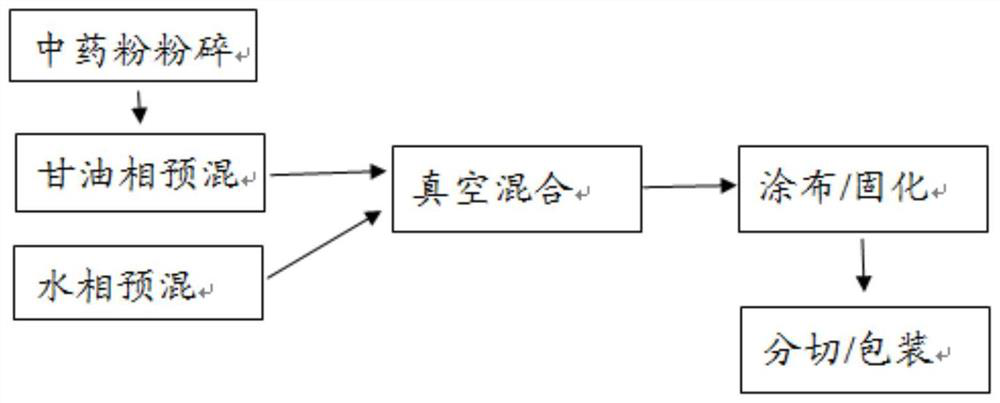
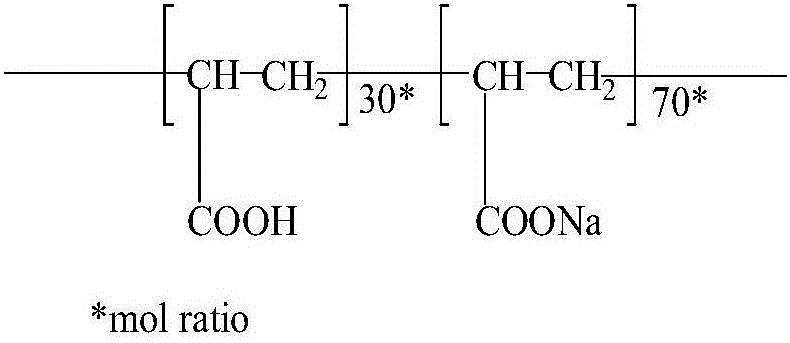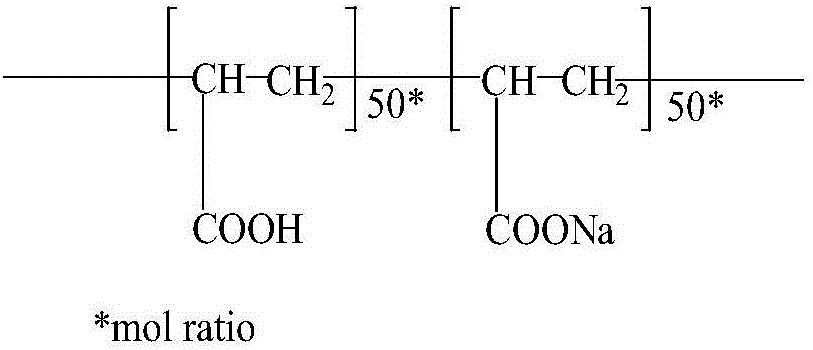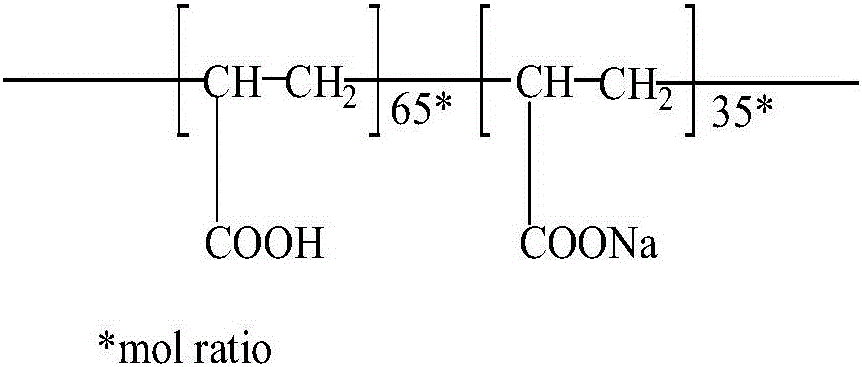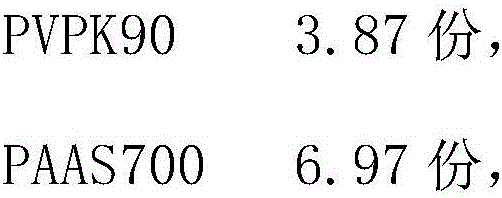Patents
Literature
50 results about "Dihydroxyaluminium aminoacetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical cold compress plaster and preparation process thereof
InactiveCN105878217ASimple production processEase of mass productionAntipyreticAnalgesicsTriclosanVitamin C
The invention provides a medical cold compress plaster. The medical cold compress plaster is composed of a support layer, a gel layer and an isolating layer, and has the special shape according with the physiological curve of the applied part. An oil phase and a water phase forming the hydrophilic gel layer respectively comprise the following components in percentage by weight: the oil phase: 20-35% of a dispersing agent, 5-7% of macromolecule resin, 0.05-0.15% of dihydroxyaluminium aminoacetate, 0.01-0.15% of ethylene diamine tetraacetic acid, 0.15% of absolute ethyl alcohol, 0.04-0.1% of menthol, 0.1-1% of vitamin E, and 0.05-0.1% of triclosan; the water phase: 0.2-0.8% of tartaric acid, 1.2-2% of carbomer, 0.5-1% of sodium carboxymethylcellulose, 0.01-0.1% of vitamin C, the balance of medical purified water, and the total weight percentage is 100%. A preparation process comprises the following steps: dispersing all the components of the oil phase into the dispersing agent, and uniformly stirring the components; dissolving all the components of the water phase into water, and uniformly stirring the components; mixing the water phase and the oil phase, uniformly stirring the water phase and the oil phase in a vacuum mixing pot, thus obtaining the hydrophilic gel substrate of the medical cold compress plaster, coating the support layer with the hydrophilic gel substrate, meanwhile covering with the isolating layer, and carrying out cutting, solidifying and packaging to obtain the needed medical cold compress plaster product.
Owner:HANGZHOU JIERSI BIOTECH CO LTD
Chitin gel band-aid and preparation method thereof
The invention aims at disclosing a chitin gel band-aid and a preparation method thereof. The band-aid comprises a carrier, wherein the carrier is provided with hydrogel hemostasis surgical dressing, the hydrogel hemostasis surgical dressing comprises the following components by weight percentage: 0.5-5% of chitin, 1.5-5% of sodium polyacrylate, 5-10% of sodium carboxymethylcellulose, 0.5-1% of povidone, 0.5-2% of gelatin, 0.05-0.5% of dihydroxyaluminium aminoacetate, 20-40% of glycerinum, 36-71.9% of purified water, and 0.05-0.5% of glacial acetic acid. Compared with the prior art, the chitin gel band-aid has anti-inflammatory and sterilizing functions due to the combination of the wet environment and the effect of the chitin added into the gel, so that the wound can be healed quickly, the pain of a patient is reduced, and the purpose of the chitin gel band-aid is reached.
Owner:上卫中亚卫生材料江苏有限公司
Gel emplastrum matrix and preparation method and application thereof
InactiveCN105997953AImprove initial viscosityGood formabilityPharmaceutical non-active ingredientsSheet deliveryEthylene diamineAcetic acid
The invention discloses a gel plaster base, which comprises the following components in parts by mass: 4.0-6.0 parts of partially neutralized sodium polyacrylate, 0.2-2.5 parts of pullulan, and 0.12-2 parts of glyoxate 0.30 parts, 20-30 parts of glycerin, 0.08-0.20 parts of disodium edetate, 0.20-0.60 parts of tartaric acid, 0.3-0.5 parts of galadan, 60-75 parts of water. The gel plaster matrix of the present invention has the advantages of high initial adhesion, good formability, good skin followability, and no irritation to the skin, and is very suitable for preparing gel plasters; meanwhile, the gel plaster of the present invention The preparation method of the ointment base is simple, easy to operate, short in time, low in energy consumption and low in cost, and is very suitable for industrial production.
Owner:成都抚南医药有限公司
Flurbiprofen cataplasm
ActiveCN106667970AFast transdermal absorptionImprove the speed of transdermal absorptionOrganic active ingredientsAntipyreticCarboxymethyl celluloseEthylene diamine
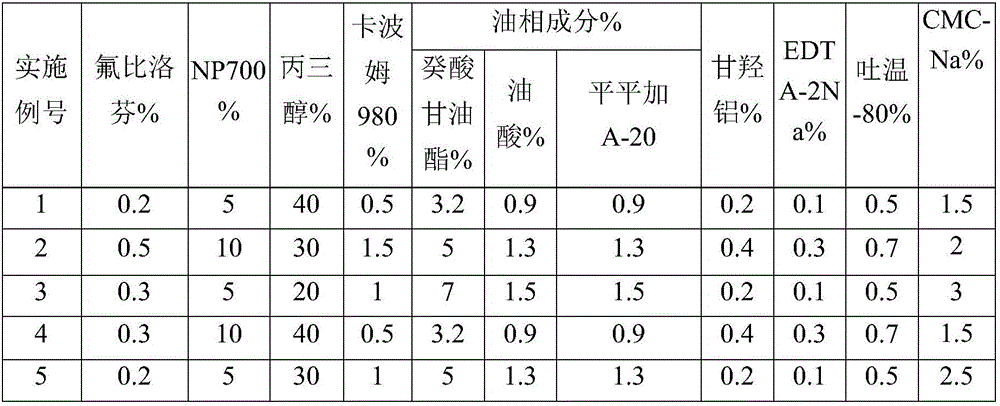
The invention discloses a flurbiprofen cataplasm, which consists of a backing layer, a medicament storage space and a protective layer, wherein the medicament storage space consists of the following components in percentage by weight: 0.2 to 0.5 percent of flurbiprofen serving as an active ingredient, 5 to 10 percent of an oil-phase component, 5 to 10 percent of partial neutralization sodium polyacrylate, 20 to 40 percent of a humectant, 0.5 to 1.5 percent of carbomer 980, 1.5 to 3 percent of CMC-Na (sodium carboxymethyl cellulose), 0.2 to 0.4 percent of dihydroxyaluminium aminoacetate, 0.1 to 0.3 percent of EDTA-2Na (disodium ethylene diamine tetraacetic acid, 0.5 to 0.7 percent of tween 80, 1 to 3 percent of a filler and the balance of water, wherein the oil-phase component is prepared from caprin, oleic acid and peregal A-20 in a mass ratio of 1:(0.2 to 0.3):(0.2 to 0.3), and the partial neutralization sodium polyacrylate, the humectant, the carbomer 980, the CMC-Na, the dihydroxyaluminium aminoacetate, the EDTA-2Na and the tween 80 form a water-phase component.
Owner:北京茗泽中和药物研究有限公司
Electric hydrogel sticking agent for instantaneous pulse electric field transdermal drug administration and preparation method thereof
InactiveCN101926784AHigh drug loadingImprove conductivitySurgical adhesivesInorganic non-active ingredientsOral medicineLiver and kidney
The invention discloses an electric hydrogel sticking agent for instantaneous pulse electric field transdermal drug administration and a preparation method thereof. The preparation method comprises the following steps of: weighing and evenly mixing NP-700, NP-800, PVP-k90, silver ion, EDTA, dihydroxyaluminium aminoacetate and kaolin, adding the mixture into glycerol with the prescription quantity, evenly stirring to obtain glycerol phase, weighing the tartaric acid with the prescription quantity, dissolving in the water to obtain water phase, adding the water phase into the glycerol phase, adding the Chinese traditional medicine extractive with the prescription quantity, stirring to obtain the grease of the Chinese traditional medicine electric hydrogel sticking agent, and coating the grease on a medical non-woven fabric, cutting, statically crosslinking and packing. The electric hydrogel sticking agent has the characteristics of large medicine loading rate, good electric performance, no stimulation, no anaphylaxis and comfortable application, can promote the transdermal absorption of multi-components of the Chinese traditional medicine by combining with the instantaneous pulse electric field, and solves the problem of the inconvenience of the drug administration of the patient who can not continuously administrate the drug caused by the gastrointestinal tract adverse reaction and the damage to the liver and kidneys of the long-term oral medicine.
Owner:NORTHWEST UNIV
Patch capable of calming nerves and helping sleep and preparation method of patch
The invention discloses a patch capable of calming nerves and helping sleep. The patch capable of calming nerves and helping sleep comprises a backing layer, a polymer gel layer and an anti-sticking layer, wherein the anti-sticking layer is formed by a polyethylene film and is arranged on the upper layer of the polymer gel layer; the backing layer is arranged on the lower layer of the polymer gel layer; the polymer gel layer comprises the following components in percentage by weight: 30-32% of a polymer gel material, 0.07-0.08% of dihydroxyaluminium aminoacetate, 0.5-1.5% of lavender essential oil, 0.5-1.5% of rose essential oil and purified water which is added until the percentage by weight is 100%; the polymer gel material comprises a dispersing agent, a wetting agent, a cross-linking agent, an adhesive and a thickening agent; the preparation method of the patch capable of calming nerves and helping sleep comprises the following steps: adding 24.5-25% of glycerol and 0.07-0.08% of dihydroxyaluminium aminoacetate into 2.7-3.1% of sodium polyacrylate and stirring for 20 minutes; adding 0.2-0.4% of polyvinylpolypyrrolidone, 2.3-3% of polyvinylpyrrolidone, 0.3-0.5% of sorbitol and purified water, and vacuum stirring for 5-8 minutes; adding active materials comprising 0.5-1.5% of rose essential oil and 0.5-1.5% of lavender essential oil, and vacuum stirring for 5 minutes. The preparation method of the patch capable of calming nerves and helping sleep is convenient in process; the prepared patch is convenient to use; the requirements of daily life are met.
Owner:施敬东
Day and night fever-cooling patches
InactiveCN104547023AWith evacuation of wind and heatHeat-clearing and detoxifyingAntipyreticAnalgesicsGlycerolHeadaches
Owner:GUANGZHOU JIURUN BIOTECH CO LTD
Method for preparing compound aspirin double-layer tablet
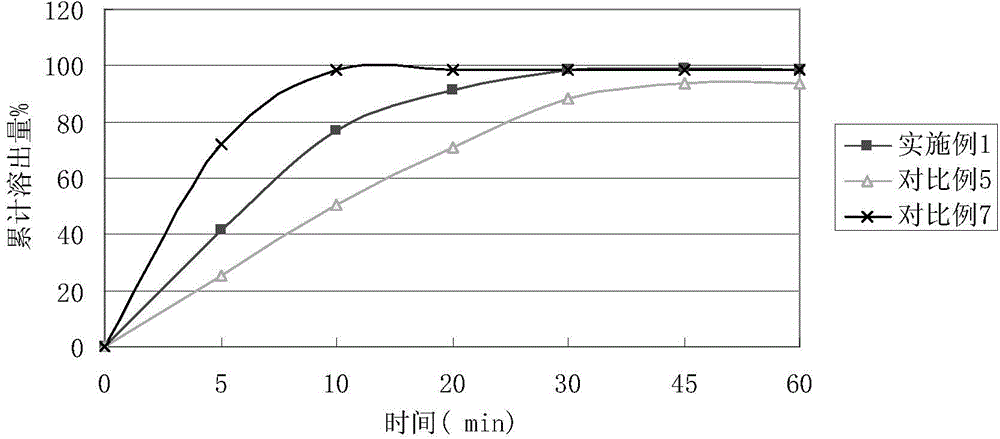
ActiveCN104434957AGood dissolution effectPromote absorptionOrganic active ingredientsAntipyreticPolymer scienceLow-substituted hydroxypropylcellulose
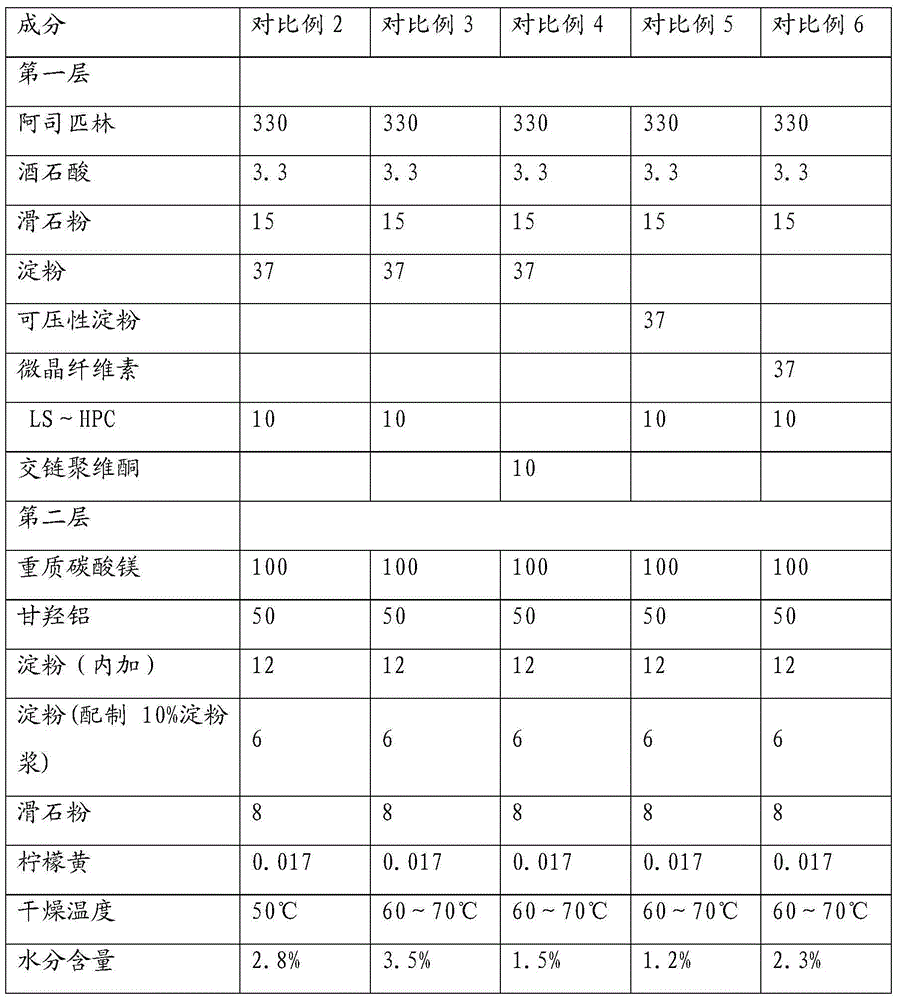
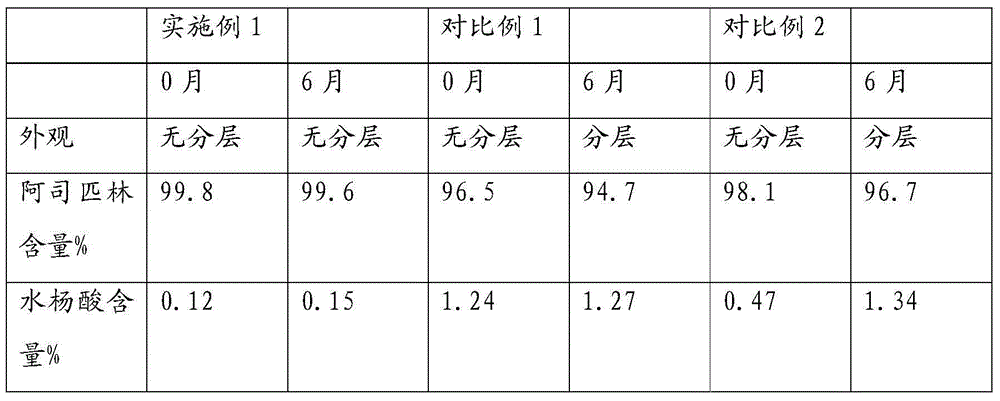
The invention relates to a method for preparing a compound aspirin double-layer tablet. The method is characterized in that aspirin, tartaric acid, low-substituted hydroxypropyl cellulose, starch and talcum powder are prepared into a first layer, and while heavy magnesium carbonate, dihydroxyaluminium aminoacetate, starch, lemon yellow and talcum powder are prepared into a second layer, and then the first layer and the second layer are pressed into the tablet. The compound aspirin tablet prepared by the method is smooth in surface, free of layering, and high in stability.
Owner:TIANSHENG PHARMA GROUP
Cataplasma for treating body surface infectious diseases
InactiveCN102641371AEasy to useComfortable to useAntiinfectivesPharmaceutical non-active ingredientsSide effectGlycerol
Provided is cataplasma for treating body surface infectious diseases applied to the technical field of medicines. The product prescription comprises cortex phellodendri chinensis, calcined gypsum, sodium polyacrylate, peach gum, polyvinylpyrrolidone, sodium carboxymethylcellulose, kaolin, glycerol, dihydroxyaluminium aminoacetate, tartaric acid, azone, diethylamine tetraacetic acid disodium and water. The preparation method includes solving the polyvinylpyrrolidone in water completely, adding the sodium carboxymethylcellulose and the peach gum into the mixture, mixing the mixture evenly, continuously adding the tartaric acid to obtain mixing liquid 1, namely water phase, placing the sodium polyacrylate, the diethylamine tetraacetic acid disodium, the dihydroxyaluminium aminoacetate, the azone and the kaolin in the glycerin, stirring the mixture to enable the mixture to be completely swelled, then adding the cortex phellodendri chinensis and the calcined gypsum, evenly stirring the mixture to obtain mixing liquid, namely oil phase 2, adding the water phase into the oil phase, completely stirring the mixture till the mixture is even, coating paste on medical non-woven fabric through a compression molding method, conducting film bonding and slice cutting, and placing the mixture to conduct complete crosslinking to obtain the cataplasma. The cataplasma is simple in using method, suitable for being applied in summer, free of side effects, good in air permeability, free of pollution to clothes and free of residual. Users do not have uncomfortable feeling if using the cataplasma, and the cataplasma has no stimulation and anaphylaxis on skins.
Owner:FIRST AFFILIATED HOSPITAL OF LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Transdermal patch containing diclofenac epolamine and preparation method thereof
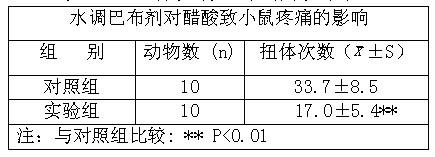
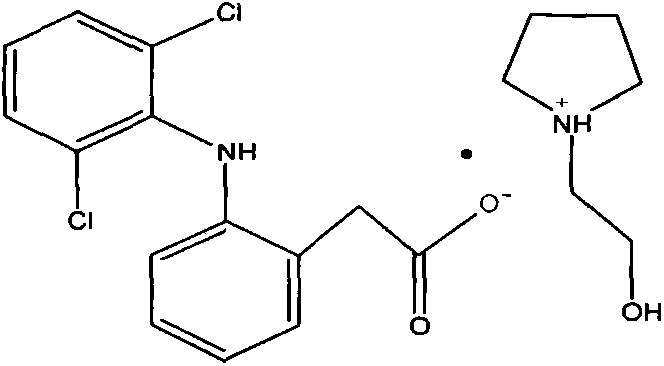
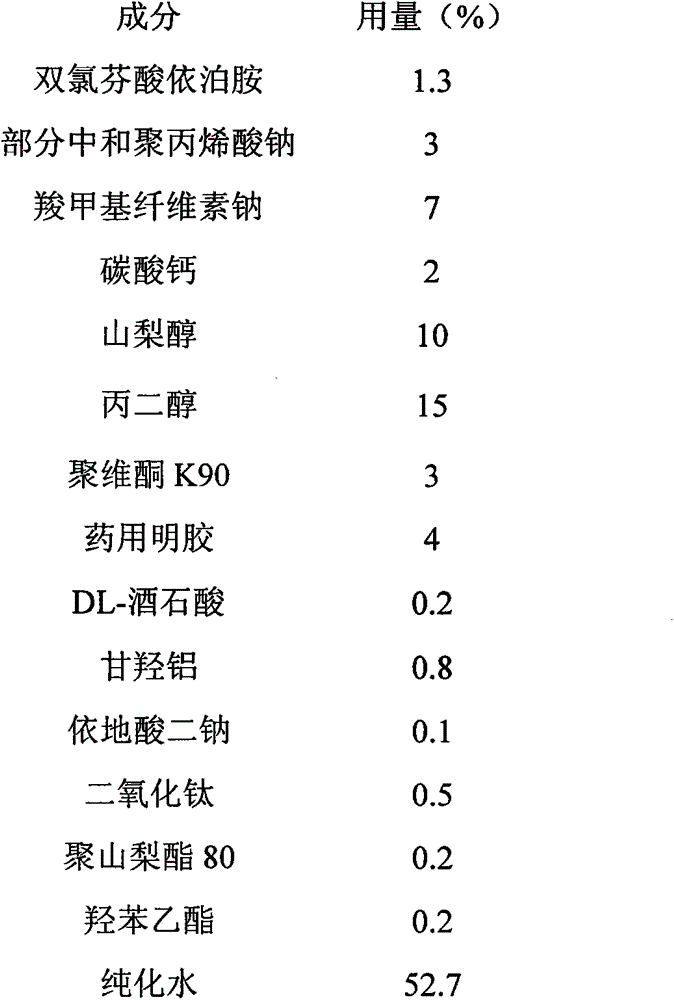
The invention provides a transdermal patch containing diclofenac epolamine and its preparation method. The transdermal patch contains the following ingredients (by weight): 0.5-5.0% (preferably 1.3%) of diclofenac epolamine which is used as a main active ingredient, 1-10% of a skeleton agent, 15-25% of a wetting agent, 0-0.5% of a complexing agent, 0-10% of an adhesive, 0-1% of a whitening agent, 0-1% of a skin penetration enhancer, 0-1% of a bacteriostat, 0-1% of a crosslinking regulator, etc. and the rest of purified water. in the preparation, dihydroxyaluminium aminoacetate is selected as the crosslinking regulator, and ethylparaben is selected as the bacteriostat. The diclofenac epolamine transdermal patch prepared by the technology has more stable quality, is convenient to use, has a simple preparation process and is suitable for large-scale production.
Owner:NANJING HEALTHNICE MEDICAL TECH
Traditional Chinese medicine cataplasma for treating arthralgia and myalgia and preparation method thereof
InactiveCN107693688ASignificant effectSimple production methodAntipyreticAnalgesicsJoint arthralgiaAconite Root
The invention provides a traditional Chinese medicine cataplasma for treating arthralgia and myalgia. The traditional Chinese medicine cataplasma is composed of non-woven fabrics, an antiadherent filmand a medicine containing ointment. The medicine containing ointment comprises Chinese angelica, banksia rose, polyghace seche, Radix Astragali, Radix Codonopsis, Rhizoma Paridis, gentrin knotweed, cassia twig, wolfberry, bark of ash, radix aconiti preparata, wild aconite root, Himalayan teasel root, cinnamon, safflower, polyisobutylene, dihydroxyaluminium aminoacetate, EDTA-2Na, sodium polyacrylate, carbomer, glycerin, Arabic gum, polyvinylpyrrolidone, borneol, menthol, purified water and ethanol. A preparation method of the traditional Chinese medicine cataplasma comprises the following steps: firstly extracting volatile oil and an extract respectively; then preparing a matrix; adding borneol and menthol and the extract and volatile oil into the matrix; uniformly stirring; coating; slicing; and packaging to obtain the cataplasma. The cataplasma of the invention has remarkable curative effects on arthralgia and myalgia, numbness of limbs and rheumatic arthritis, is not easy to causeirritability, has a simple production method, and is suitable for industrial mass production.
Owner:JIANGSU 707 NATURAL PHARMA
Cold-compression patch with hydrogel as substrate and method for manufacturing cold-compression patch
InactiveCN105078645AGood compatibilityLong moisturizing periodAntipyreticAnalgesicsAlcoholIrritation
The invention discloses a cold-compression patch with hydrogel as a substrate and manufacture of the cold-compression patch. The cold-compression patch comprises, by weight, 0.2-3 parts of peppermint, 5-30 parts of glycerin, 0.1-1 part of ethylparaben, 0.2-2 parts of dihydroxyaluminium aminoacetate, 5-20 parts of NP-700, 1-5 parts of ethyl alcohol, 35-75 parts of purified water, 2-25 parts of K-90, 1-10 parts of XL-10, 0.2-1 part of tartaric acid and 0.3-1 part of light brown. The cold-compression patch and the manufacture have the advantages that the hydrogel is mainly selectively made of hydrophilic substrates, and accordingly the manufactured patch is good in skin compatibility, long in moisture retention period, high in perspiration resistance and free of irritation or sensitization, and can be repeatedly stuck to patients; technologies are simple and can be carried out at the normal temperature, the cold-compression patch is convenient to manufacture, harm to operators can be prevented in integral technological procedures, discharge of waste gas, waste water and industrial residues can be prevented in the production and manufacture procedures, and accordingly the cold-compression patch is environmental friendly and safe and is free of environmental pollution.
Owner:TIANJIN ZHUOPU MEDICAL EQUIP CO LTD
Medicament matrix composition, preparation method and usage thereof
InactiveCN105919983AInitial viscosity equilibriumViscosity balancePharmaceutical non-active ingredientsSheet deliveryDiseaseUltimate tensile strength
The invention relates to a medicament matrix composition and a preparation method. PVPK90 (polyvinylpyrrolidone K90), PAAS700 (sodium polyacrylate 700), dihydroxyaluminium aminoacetate, tartaric acid, glycerol and purified water are taken as raw materials and are mixed, stirred and formed into the medicament matrix composition. The matrix has the characteristics of primary viscous force, keeping viscous force, peel strength, adhesive force balancing, and the like; the matrix is mixed with the raw material drugs; the materials are uniformly stirred and mixed, coated, dried, checked and packaged, thereby acquiring the gel plaster used for treating the related diseases.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
Pain relieving cataplasm for relaxing tendon and activation collaterals and preparation method of pain relieving cataplasm
ActiveCN104644933AImprove stabilityGood followabilityAnthropod material medical ingredientsHydroxy compound active ingredientsIrritationGlycerol
The invention discloses a pain relieving cataplasma for relaxing tendon and activation collaterals. The pain relieving cataplasma comprises 1-5 weight parts of active pharmaceutical ingredient and 30-300 weight parts of matrix, wherein the matrix is prepared from the following raw materials in parts by weight: 5-60 parts of Carbopol, 10-90 parts of partial neutrolization sodium polyacrylate, 0.1-2 parts of ethylenediamine tetraacetic acid, 5-80 parts of povidone K30, 10-120 parts of gelatin, 60-180 parts of glycerinum, 1-5 parts of ethylparaben, 0.1-2 parts of dihydroxyaluminium aminoacetate, 0.05-1 part of citric acid, 1-8 parts of ethyl alcohol and 150-300 parts of water. The invention further discloses a preparation method for the pain relieving cataplasmata, the pain relieving cataplasmat for relaxing tendon and activation collaterals prepared by the method is simple in process and safe in production, has no pollution to the environment, the efficacy of the pain relieving cataplasma is obvious, and the pain relieving cataplasmat generates little irritation and allergy to the skin.
Owner:ANHUI ANKE YULIANGQING PHARMA
Medicinal preparation of extractive from compound aspirin and rice fermentred with red yeast
InactiveCN1660321ARelieve irritationCoronary heart disease remissionMetabolism disorderPharmaceutical delivery mechanismAspirinYeast
Owner:FUKANGREN BIO PHARMA
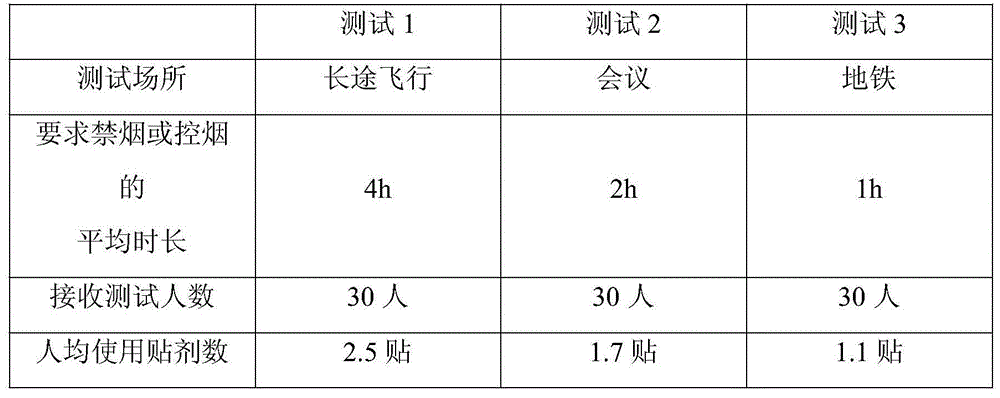
Hydrogel emergency tobacco control patch formula and method for preparing tobacco control patch
InactiveCN104940408APromote softeningImprove stabilityOrganic active ingredientsNervous disorderTobacco controlGlycerol
The invention discloses a hydrogel emergency tobacco control patch formula and a method for preparing a tobacco control patch. The method for preparing the tobacco control patch comprises the steps that hydrogel is prepared by mixing 0.1%-2.0% of natural tobacco extracts, 1%-15% of nicotine clathrate compound, 0.5%-3.0% of mint extracts, 5.0%-10.0% of sodium polyacrylate, 0.5%-3.0% of sodium carboxymethylcellulose, 0.1%-2.0% of dihydroxyaluminium aminoacetate, 0.2%-1.0% of dihydroxysuccinic acid, 4.0%-8.0% of ethyl alcohol, 20.0%-30.0% of glycerinum and 45.0%-60.0% of purified water, and then the hydrogel is smeared on base cloth. The tobacco control patch prepared through the method is low in nicotine content, long in stipulated use time, good in even slow-release effect, safe, controllable, high in skin friendliness, good in adhering performance and convenient to carry and use, the preparation technique is simple, secondary pollution to the environment is avoided, and the tobacco addiction can be released and controlled.
Owner:云南拓宝科技有限公司
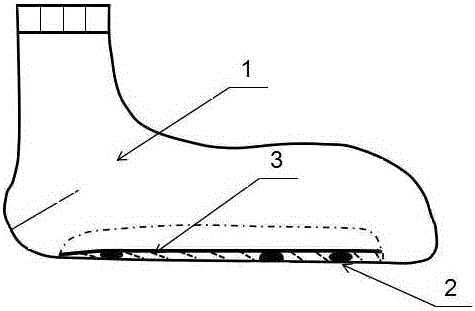
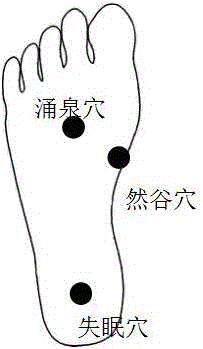
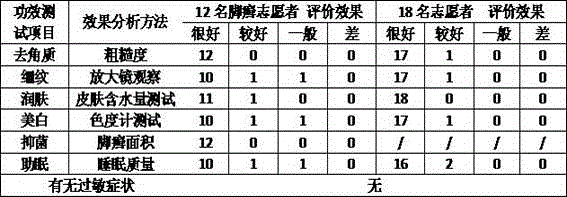
Gel magnetic therapy massage sock and method for manufacturing same
InactiveCN106072804APromote circulationImprove sleepingElectrotherapyDevices for pressing relfex pointsJojoba oilInsomnia
The invention discloses a gel magnetic therapy massage sock and a method for manufacturing the same. The gel magnetic therapy massage sock comprises a sock, magnetic therapy massage points and a gel layer. Strong-intensity magnets are arranged on the sock to form the magnetic therapy massage points and correspond to the Yongquan points, the insomnia points and the Rangu points of the foot soles of users; the gel layer is arranged at the bottom of an inner layer of the sock and covers the magnetic therapy massage points. The gel layer comprises, by weight, 0.01-0.10% of chitosan oligosaccharide, 0.1-0.3% of tartaric acid, 20.0-35.0% of glycerin, 0.05-0.35% of dihydroxyaluminium aminoacetate, 0.03-0.15% of EDTA (ethylene diamine tetraacetic acid), 3.0-9.0% of AP-800 surfactants, 0.5-3.0% of absolute ethyl alcohol, 1.5-2.5% of jojoba oil, 1.0-2.0% of grape seed oil, 0.05-0.1% of tea tree oil, 0.01-0.02% of rose oil, 0.01-0.02% of jasmine essential oil, 0.02-0.05% of sweet almond oil, 0.03-0.05% of lavender oil, 0.1-0.5% of lemon essential oil and the balance purified water. The gel magnetic therapy massage sock and the method have the advantages that skin moistening, whitening, exfoliating, wrinkle reducing, crack preventing and treating and antibacterial effects can be realized by the gel magnetic therapy massage sock, the blood circulation further can be promoted, the gel magnetic therapy massage sock is beneficial to sleep and can be reused for a long time, and aging can be prevented.
Owner:QINGDAO MINGYAOTANG MEDICAL TECH DEV
Physique cold compress gel and preparation method thereof
InactiveCN108969619AImprove complianceGood drug releaseHeavy metal active ingredientsHydroxy compound active ingredientsSide effectMyrrh
The invention discloses physique cold compress gel and a preparation method thereof. The physique cold compress gel is prepared from a raw material drug and an accessory drug; the raw material drug isprepared from the following medicinal materials, in parts by weight: 72-78 parts of resina draconis, 72-78 parts of radix notoginseng, 47-53 parts of frankincense, 56-62 parts of myrrh, 46-52 parts of flos carthami, 8-12 parts of calcined native copper, 14-17 parts of velvet antler, 48-52 parts of rhizoma cibotii, 48-50 parts of cortex acanthopanacis, 48-52 parts of radix dipsaci and 3-6 parts ofborneol; the accessory drug is prepared from, in parts by weight: 1-10 parts of carbomer, 5-15 parts of triethanolamine, 1-10 parts of azone, 5-15 parts of twain-80, 400-500 parts of glycerinum, 20-100 parts of sodium polyacrylate, 20-100 parts of kaolin, 1-10 parts of dihydroxyaluminium aminoacetate, 0.5-20 parts of ethylene diamine tetraacetic acid and 0.5-20 parts of tartaric acid. The prepared gel has no irritation and allergenicity to the skin, does not pollute the clothes, has no residual, has the good drug releasing performance, and can continuously release the drug; and compared withthe prior art, the gel has the characteristics that the curative effects are precise, the safety is good, no side effects exists, application is convenient, the cost is low, the effect is superior tothe prior art.
Owner:穆艳华
Preparation method and application of PAMs (natural plant antimicrobial solution) hydrogel patch
ActiveCN108853369AGuaranteed efficient deliveryGuaranteed effective deliveryAntibacterial agentsAerosol deliveryGlycerolTherapeutic effect
The invention discloses a preparation method and application of PAMs (natural plant antimicrobial solution) hydrogel patch. The hydrogel patch is prepared from 15-45 parts by weight of glycerol, 2-6 parts by weight of NP-700, 0.2-0.6 parts by weight of dihydroxyaluminium aminoacetate, 13.6-40.7 parts by weight of distilled water, 0.1-0.3 parts by weight of tartaric acid, 1.5-4.5 parts by weight ofazone, 1.5-4.5 parts by weight of propylene glycol, 0.25-0.75 parts by weight of SDS (sodium dodecyl sulfate), and 15.85-47.55 parts by weight of PAMs. The hydrogel patch provided by the invention has the beneficial effects of good moldability, viscoelasticity, gas permeability, good peeling property from skin and good therapeutic effect on skin burns, and can prevent skin ulceration, and has good antimicrobial and anti-inflammatory effects on wounds and good repairing effects on wound surface to slow down the problem of scar residue. The hydrogel patch is obtained by one-time heat preservation crosslinking, and the operation is convenient and advantageous for large-scale production.
Owner:云南民族医药研究所有限公司
Aluminum, magnesium and aspirin tablet (II) and preparing method thereof
ActiveCN106727380AAdvanced preparation technologyGood curative effectOrganic active ingredientsPill deliveryDissolutionSilicon dioxide
The invention belongs to the technical field of pharmacy, and particularly relates to an aluminum, magnesium and aspirin tablet (II) and a preparing method thereof. The aluminum, magnesium and aspirin tablet (II) is made by tableting an aspirin layer and a buffering layer, and the aspirin layer is prepared from aspirin, mannitol, corn starch, tartaric acid, gelatin, tartrazine aluminum lake and silicon dioxide; the buffering layer is prepared from dihydroxyaluminium aminoacetate, heavy magnesium carbonate, mannitol, corn starch, gelatin and silicon dioxide. The aluminum, magnesium and aspirin tablet (II) prepared from the preparing technology has a better dissolution rate, and the impurities are obviously lowered compared with an impurity index in an existing product.
Owner:中健康桥医药集团股份有限公司 +1
Gel plaster for treating dysmenorrhea and preparation method of gel plaster
InactiveCN109331159AHigh extraction rateGood initial adhesionUnknown materialsPharmaceutical non-active ingredientsMyrrhAlcohol
The invention belongs to the technical field of pharmaceutical preparations containing radix angelicae sinensis and particularly relates to gel plaster for treating dysmenorrhea. The gel plaster is prepared from the following components: carbomer, sodium polyacrylate, dihydroxyaluminium aminoacetate, kaolin, glycerol, tartaric acid and an extract; a preparation method of the extract comprises thefollowing steps: adding radix angelicae sinensis, rhizoma chuanxiong, cortex cinnamomi, fructus foeniculi, myrrh and rhizoma zingiberis into water, soaking and carrying out steam distillation and extraction to obtain volatile oil, herb residues and an extracting solution A; adding radix paeoniae rubra, rhizoma corydalis, pollen typhae, trogopterus dung, cortex cinnamomi and the herb residues intoan ethanol solution for extracting, recovering the ethanol in the extracting solution until no alcohol taste is produced to obtain an extracting solution B; combining and concentrating the extractingsolution A and the extracting solution B to obtain an initial extract; then adding the volatile oil and uniformly mixing, thus obtaining a finished product.
Owner:谈宗华
Compound aspirin tading kind medicinal preparation and its application
InactiveCN1651085AImprove stabilityInhibitory activityOrganic active ingredientsMetabolism disorderAspirinStatine
Owner:FUKANGREN BIO PHARMA
Sachet sticker with antibacterial, antiviral and nerve-calming effects and preparation method of sachet sticker
InactiveCN111481643AImprove securityAntibacterialAntibacterial agentsBiocideBiotechnologySandalwood oil
The invention provides a sachet sticker with antibacterial, antiviral and nerve-calming effects and a preparation method thereof, and belongs to the technical field of spices, essences and daily chemicals, the sachet sticker comprises sodium polyacrylate, polyacrylamide, tartaric acid, dihydroxyaluminium aminoacetate, glycerin, water, povidone, Tween 80 and aromatic components; the aromatic component comprises traditional Chinese medicine powder, a plant extract and essential oil; wherein the traditional Chinese medicine powder comprises chrysanthemum powder, rose flower powder and mint powder; the plant extract comprises a kaempferol extract, a perilla alcohol extract and an agastache rugosus alcohol extract; the essential oil comprises lavender oil, sandalwood oil, blumea oil, atractylodes lancea oil and grassleaf sweelflag rhizome oil. The sachet paste adopts a gel paste product form, can be pasted on a mask, a collar, cuffs, a nose bridge, ear peripheries, a forehead and other parts in use, has antibacterial, antiviral and nerve-calming effects, and is long in lasting time.
Owner:广州市和一医疗科技有限公司
Hydrogel magnet therapy traditional Chinese medicine plaster and preparation method thereof
InactiveCN111658716AImprove water absorptionImprove breathabilityElectrotherapyHydroxy compound active ingredientsMyrrhPolyvinyl alcohol
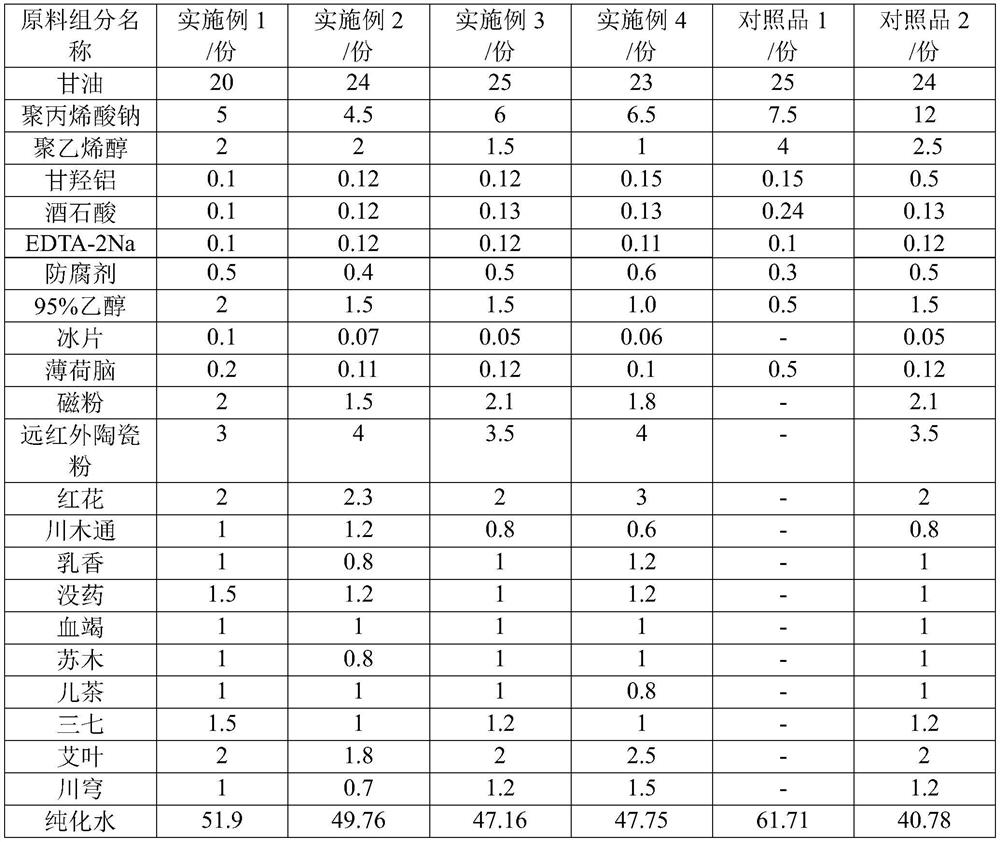
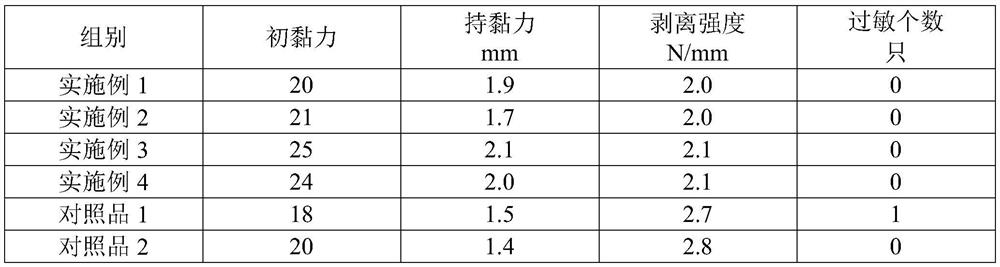
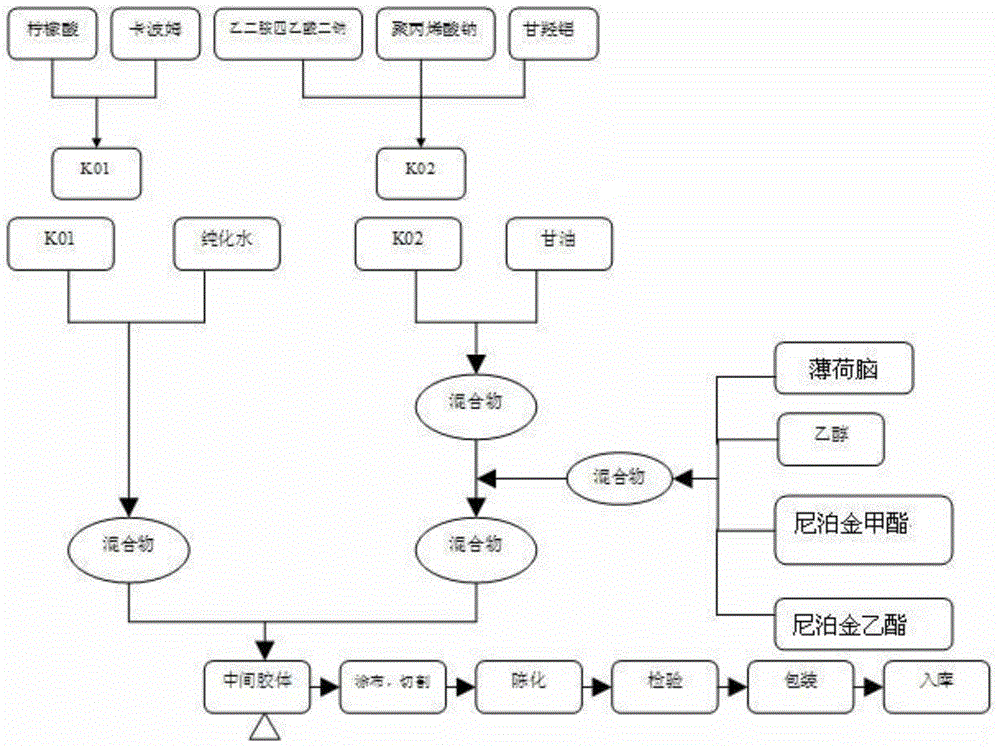
The invention discloses a hydrogel magnet therapy traditional Chinese medicine plaster and a preparation method thereof. The hydrogel magnet therapy traditional Chinese medicine plaster comprises thefollowing components in parts by mass: 15-25 parts of glycerol, 1-10 parts of sodium polyacrylate, 1-10 parts of polyvinyl alcohol, 0.1-2 parts of dihydroxyaluminium aminoacetate, 0.1-2 parts of tartaric acid, 0.1-2 parts of EDTA-2Na, 0.9-9 parts of ethanol, 0.02-0.2 part of borneol, 0.02-0.2 part of menthol, 1-3 parts of magnetic powder, 2-5 parts of far infrared ceramic powder, 10-20 parts of atraditional Chinese medicine composition and 40-70 parts of water. The traditional Chinese medicine composition is prepared from at least one of flos carthami, caulis clematidis armandii, frankincense, myrrh, dragon's blood, sappan wood, catechu, radix notoginseng, rhizoma chuanxiong and folium artemisiae argyi. The hydrogel magnet therapy traditional Chinese medicine plaster disclosed by the invention has dual effects of medical therapy and magnet therapy, and has good binding power, high use comfort and no irritation to skin.
Owner:BEOGENE BIOTECH GUANGZHOU
Novel plaster and preparation method thereof
InactiveCN104622621ADoes not affect movementEasy to carryTherapeutic coolingTherapeutic heatingMentholAcetic acid
The invention discloses novel plaster and a preparation method of the novel plaster. The plaster comprises, by weight percentage, 75 percent of purified water, 0.18 percent of citric acid, 1.03 percent of carbomer, 0.03 percent of ethylenediamine tetraacetic acid disodium, 3.65 percent of sodium polyacrylate, 0.4 percent of dihydroxyaluminium aminoacetate, 0.1 percent of methylparaben, 0.1 percent of ethylparaben, 18.5 percent of glycerin, 0.7 percent of ethyl alcohol, 0.3 percent of menthol and 0.01 percent of erioglaucine. The novel plaster is convenient to prepare, comfortable and long-acting.
Owner:TIANJIN LIQI PHARMA CO LTD
Preparation method of Bitongxiao cataplasm
The invention belongs to the field of medicine processing, and specifically relates to a preparation method of a Bitongxiao cataplasm. The Bitongxiao cataplasm provided by the invention is safe, and is convenient to apply. The Bitongxiao cataplasm provided by the invention is prepared from: 1-6% of PANa, 0.1-1.2% of carbomer, 1-12% of CMC-Na, 2-8% of PVP-K30, 1-10% of gelatin, 0.8-1.4% of tartaric acid, and 0.4-0.7% of dihydroxyaluminium aminoacetate.
Owner:孔祥文
Novel nasal strip gel layer, novel nasal strip and preparation method of nasal strip
ActiveCN103191184BSuitable for useSignificant effectHydroxy compound active ingredientsRespiratory disorderSide effectGlycerol
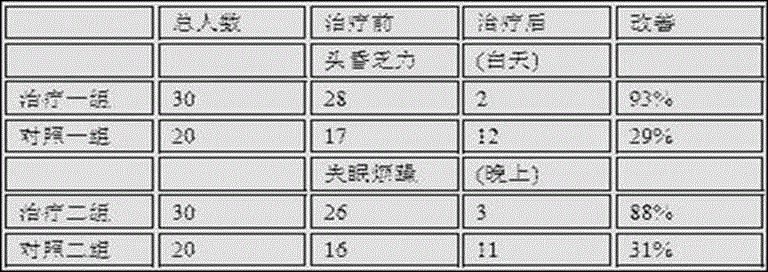
The invention provides a novel nasal strip gel layer. The novel nasal strip gel layer mainly consists of the following components in percentage by weight: 0.01-20% of peppermint and borneol mixture, 5.5% of glycerol 25% hydrogel resin, 0.12% of ethylene diamine tetraacetic acid (EDTA) disodium salt, 0.2% of dihydroxyaluminium aminoacetate, 6% of 95% ethanol, 8% of PVPK 120, 2.5% of 10% tartaric acid solution and the balance of purified water. Furthermore, the invention discloses a novel nasal strip and a preparation method of the nasal strip. According to clinical test, the nasal strip provided by the invention is remarkable in treating effect on chronic rhinitis and allergic rhinitis, mainly reflecting on an aspect of improving various symptoms of the rhinitis; and the nasal strip is concrete in treating effect, free from side effect, and applicable to various patients.
Owner:王怀伟
Oxybutynin transdermal plaster and preparation method thereof
ActiveCN113143891AHigh porosityImprove breathabilityOrganic active ingredientsAntipyreticPectinaseCellulose
The invention relates to the technical field of pharmaceutical preparations, and discloses an oxybutynin transdermal plaster and a preparation method thereof. The oxybutynin transdermal plaster comprises a backing layer and an ointment-containing body, wherein the ointment-containing body comprises the following raw materials in percentage by mass: 3-5% of oxybutynin and / or pharmaceutically acceptable salt thereof, 10-15% of sodium polyacrylate, 5-8% of pre-crosslinked pectin, 1-2% of hydroxypropyl methylcellulose, 0.5-1% of dihydroxyaluminium aminoacetate, 1.5-3.5% of pectinase @ ethyl cellulose / chitosan microcapsule, 10-15% of sorbitol, 1-2% of laurinol azone, 10-15% of ethanol and the balance water; and the pH value of the ointment-containing body is controlled to be 5.0-6.0 by a pH regulator. The oxybutynin transdermal plaster disclosed by the invention has relatively high air permeability, and can promote the release of oxybutynin by slowly releasing pectinase in the later period of application, thereby prolonging the drug effect duration and application time of the plaster.
Owner:杭州仁德药业股份有限公司
A kind of aluminum-magnesium-pirin tablet (ii) and preparation technology thereof
ActiveCN106727380BAdvanced preparation technologyGood curative effectOrganic active ingredientsPill deliveryDissolutionSilicon dioxide
Owner:中健康桥医药集团股份有限公司 +1
Anti-inflammatory and analgesic gold bone patch
InactiveCN112043780AWith expelling wind and dampnessAlleviate cervical syndromeAntipyreticAnalgesicsGlycerolInflammation resolution
The invention discloses an anti-inflammatory and analgesic gold bone patch which comprises a backing layer, a hydrogel paste layer and an anti-sticking layer. Paste in the hydrogel paste layer comprises the components in weight of 100g: 65-80g of traditional Chinese medicine extract, 3-8g of sodium polyacrylate, 1-5g of polyacrylic acid, 0.01-0.2g of dihydroxyaluminium aminoacetate, 0.5-3g of carbomer, 1-5g of polyvinyl alcohol, 0.1-1g of medical urea, 1-5g of propylene glycol, 10-20g of glycerinum, 0.01-0.3g of organic acid and 0.1-0.6g of preservative. The traditional Chinese medicine extract is prepared from eight traditional Chinese medicinal materials including radix clematidis, caulis spatholobi, caulis sinomenii, rhizoma dioscoreae nipponicae, radix et caulis flemingiae, all-grass of nippon dinquefoil, herba epimedii and radix achyranthis bidentatae, and is used in the hydrogel paste layer of the gold bone patch, the traditional Chinese medicinal materials supplement one another, adhesion and edema of damaged muscle groups are eliminated by promoting blood circulation to remove blood stasis, dispelling wind and eliminating dampness through targeted drug therapy for lesion sites, normal controllable functions of damaged nerves and damaged muscle groups are recovered, intervertebral disc degenerative changes are reversed, and normal activities of vertebral bodies and bonejoints are recovered.
Owner:贵州黔草灵生物保健研发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com