Medicinal preparation of extractive from compound aspirin and rice fermentred with red yeast
A kind of technology of aspirin and extract, applied in the field of compound pharmaceutical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
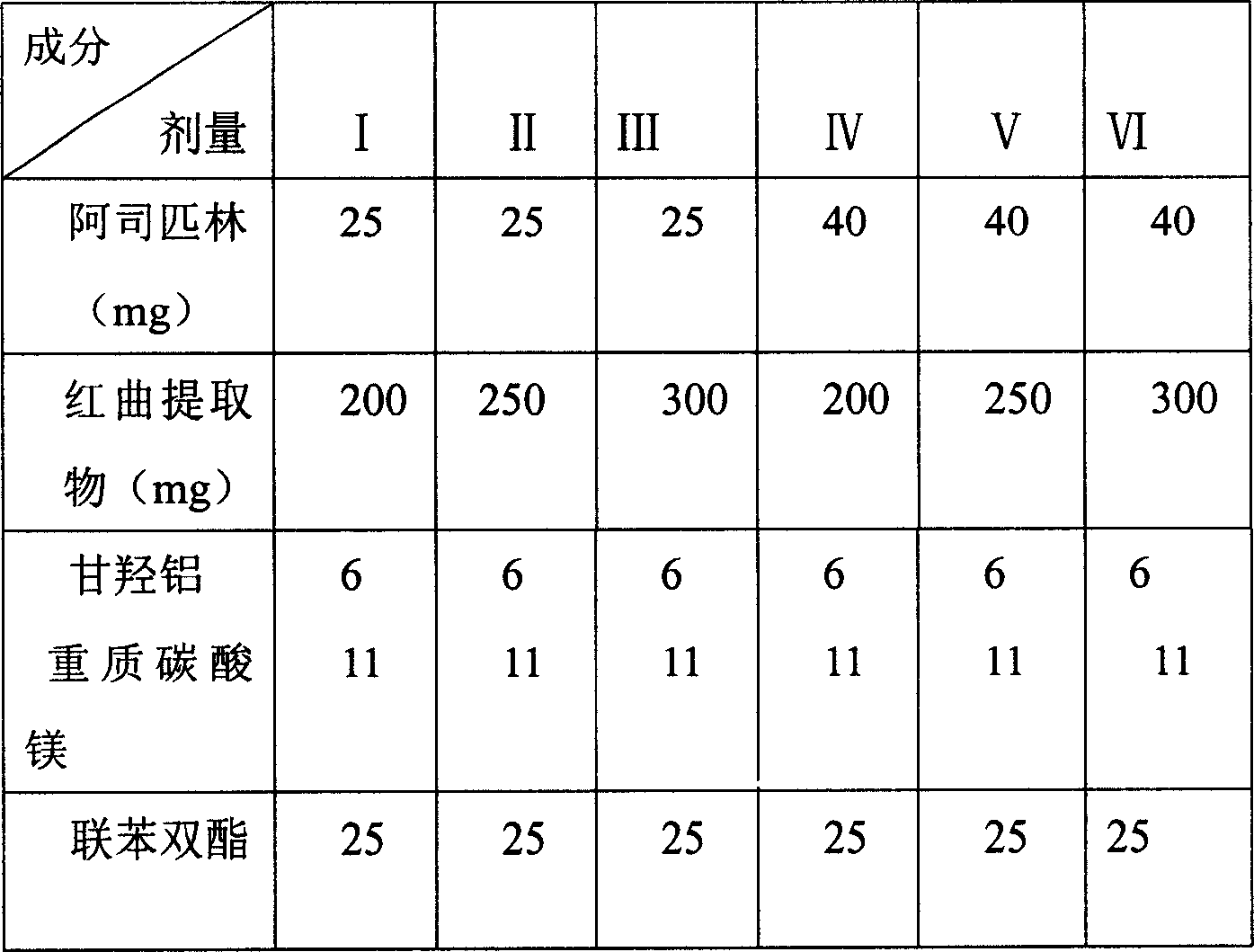
[0049] Prescription: 1000 tablets
[0050] Red Yeast Extract 300g
[0051] Aspirin 20g
[0052] Bifendate 25g
[0053] Aluminum glycylate 6g
[0054] Heavy Magnesium Carbonate 11g
[0055] Microcrystalline Cellulose 105g
[0056] Sodium carboxymethyl starch 20g
[0057] Polyvinylpyrrolidone 8g
[0058] Micronized silica gel 4g
[0060] Preparation method: Pass the medicine and auxiliary materials through 80-mesh sieve respectively, weigh the prescription amount of red yeast rice extract, aspirin, bifendate, aluminum glyoxate, heavy magnesium carbonate, microcrystalline cellulose, sodium carboxymethyl starch (2 / 3), mix evenly, make soft material with 10% polyvinylpyrrolidone aqueous solution, cross 16 mesh sieves, dry, granulate, add remaining (1 / 3) sodium carboxymethyl starch, micropowder silica gel, magnesium stearate, It can be directly compressed into tablets.
Embodiment 2
[0061] Example 2: Preparation of Red Yeast Extract+Aspirin+Malotilate Film-Coated Tablets.
[0062] prescription:
[0063] 1000 pieces
[0064] Red Yeast Extract 250g
[0065] Aspirin 25g
[0066] Aluminum glycylate 6g
[0067] Heavy Magnesium Carbonate 11g
[0068] Malotilate 100g
[0069] Microcrystalline Cellulose 67.5g
[0070] Croscarmellose Sodium 20g
[0071] 10% starch slurry appropriate amount
[0072] Micronized silica gel 5g
[0073] Magnesium Stearate 2.5g
[0074] Preparation method: pass the drug and auxiliary materials through 80 mesh sieves, weigh the prescription amount of red yeast rice extract, aspirin, aluminum glycolate, heavy magnesium carbonate, malotilate, microcrystalline cellulose, and cross-linked carboxymethyl cellulose Sodium (2 / 3), mix evenly, use 10% starch slurry to make soft material, pass through 16 mesh sieve, dry, granulate, add remaining croscarmellose sodium, micropowder silica gel, magnesium stearate and...
Embodiment 3
[0075] Example 3: Preparation of Red Yeast Extract + Aspirin + Disodium Adenosine Triphosphate Capsules
[0076] prescription:
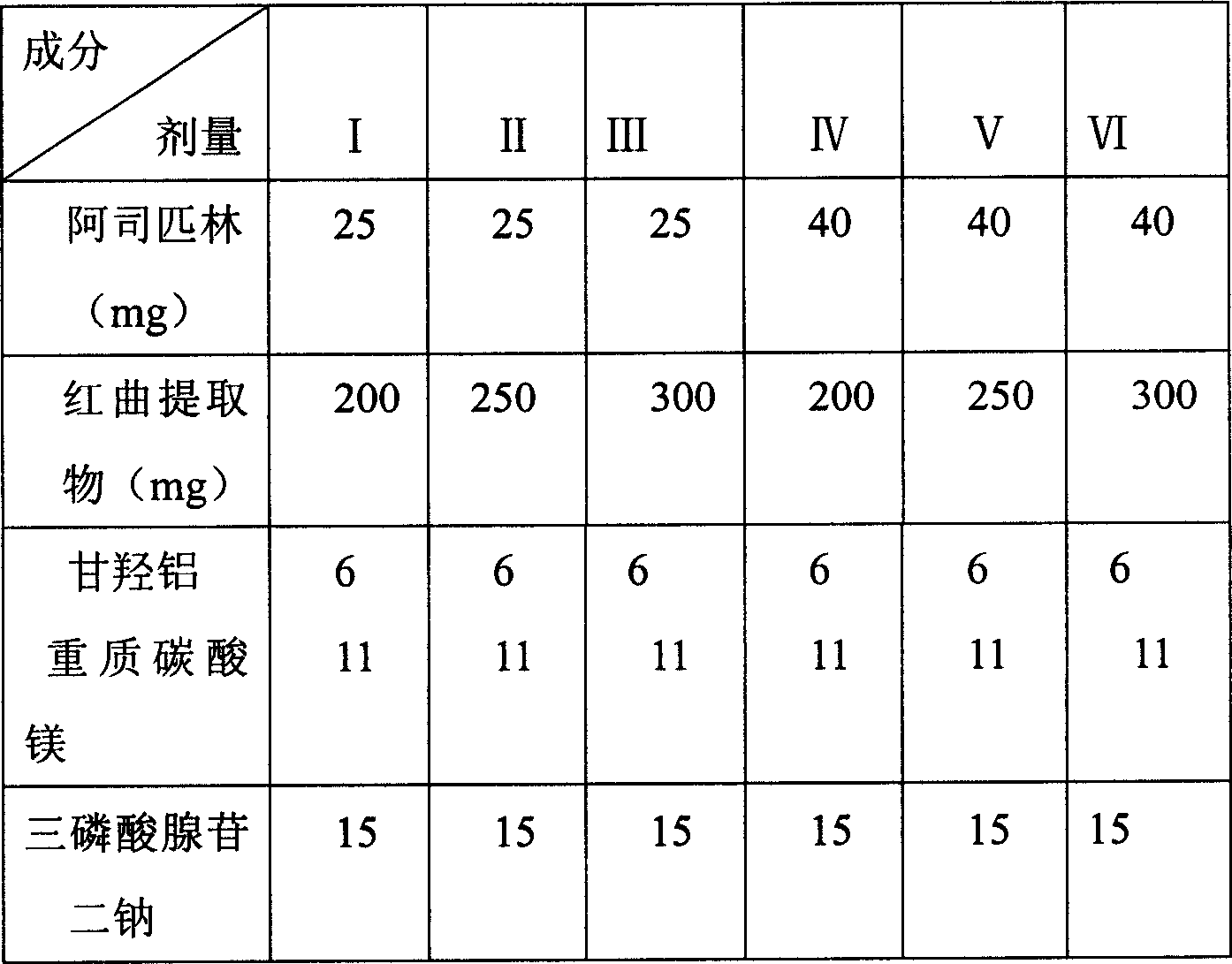
[0077] 1000 capsules
[0078] Red Yeast Extract 200g
[0079] Aspirin 40g
[0080] Aluminum glycylate 6g
[0081] Heavy Magnesium Carbonate 11g
[0082] Disodium Adenosine Triphosphate 15g
[0083] Microcrystalline Cellulose 84g
[0084] Polyvinylpyrrolidone 5g
[0085] capsule shell
[0086] Preparation method: pass the medicine and auxiliary materials through 80-mesh sieve respectively, weigh the prescription amount of red yeast rice extract, aspirin, aluminum glycolate, heavy magnesium carbonate, adenosine triphosphate disodium, microcrystalline cellulose, mix evenly, and use 5% poly The soft material is made of vinylpyrrolidone 75% ethanol solution, passed through a 16-mesh sieve, dried, granulated through a 14-mesh sieve, and the drug granules are filled into the capsule shell to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com