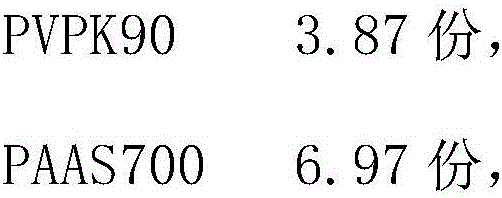Medicament matrix composition, preparation method and usage thereof
A technology of a matrix composition and a medicine is applied to the gel patch matrix composition and the field of preparing the gel patch, and can solve problems such as loss of viscosity, insufficient reflection of the quality of the gel patch, and leakage of the plaster.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Take the raw material composition of following weight:
[0049] PVPK903.87g
[0050] PAAS700 6.97g
[0051] Tartaric acid 0.25g
[0052] Aluminum glycylate 0.30g
[0053] Glycerin 35.00g
[0054] Purified water 43.61g
[0055] (2) Dissolve PVPK90 and tartaric acid in purified water, fully swell, as phase A.
[0056] (3) Mix PAAS700 and aluminum glycolate evenly, disperse them in glycerin, stir evenly at a stirring speed of 60r / min, and stir for about 15min to obtain phase B.
[0057] (4) Add phase A to phase B at one time, first stir at 60r / min, then increase the stirring speed to about 120r / min, and stir for about 15min to form a uniform paste.
[0058] (5) At 60°C, through vacuum-atmospheric pressure-vacuum three times of alternating pressure changes to remove the air bubbles in the paste, that is, the present invention has a balanced initial adhesion, adhesion, peel strength and adhesion Powerful gel patch base.
Embodiment 2
[0060] (1) Take the raw material composition of following weight:
[0061] PVPK903.80g
[0062] PAAS700 6.90g
[0063] Tartaric acid 0.24g
[0064] Aluminum glycylate 0.28g
[0065] Glycerin 34.00g
[0066] Purified water 42.35g;
[0067] (2) Dissolve PVPK90 and tartaric acid in purified water, fully swell, as phase A.
[0068] (3) Mix PAAS700 and aluminum glycolate evenly, disperse them in glycerin, stir evenly at a stirring speed of 60r / min, and stir for about 20min to obtain phase B.
[0069] (4) Add phase A to phase B at one time, first stir at 60r / min, then increase the stirring speed to about 120r / min, and stir for about 20min to form a uniform paste.
[0070] (5) At 60°C, through vacuum-atmospheric pressure-vacuum 4 times of alternating pressure changes to remove the air bubbles in the paste, that is, the present invention has a balanced initial adhesion, adhesion, peel strength and adhesion Powerful gel patch base.
Embodiment 3
[0072] (1) Take the raw material composition of following weight:
[0073] PVPK903.90g
[0074] PAAS700 7.00g
[0075] Tartaric acid 0.26g
[0076] Aluminum glycylate 0.32g
[0077] Glycerin 36.00g
[0078] Water 45.50g
[0079] (2) Dissolve PVPK90 and tartaric acid in water, fully swell, as phase A.
[0080] (3) Mix PAAS700 and aluminum glycolate evenly, disperse them in glycerin, stir evenly at a stirring speed of 60r / min, and stir for about 15min to obtain phase B.
[0081] (4) Add phase A to phase B at one time, first stir at 60r / min, then increase the stirring speed to about 120r / min, and stir for about 18min to form a uniform paste.
[0082] (5) At 60°C, through vacuum-atmospheric pressure-vacuum three times of alternating pressure changes to remove the air bubbles in the paste, that is to say, the present invention has a balanced initial adhesion, holding force, peel strength and Adhesive gel patch base.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com