Aluminum, magnesium and aspirin tablet (II) and preparing method thereof
A kind of technology of aluminum magnesium pirin tablet and aspirin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
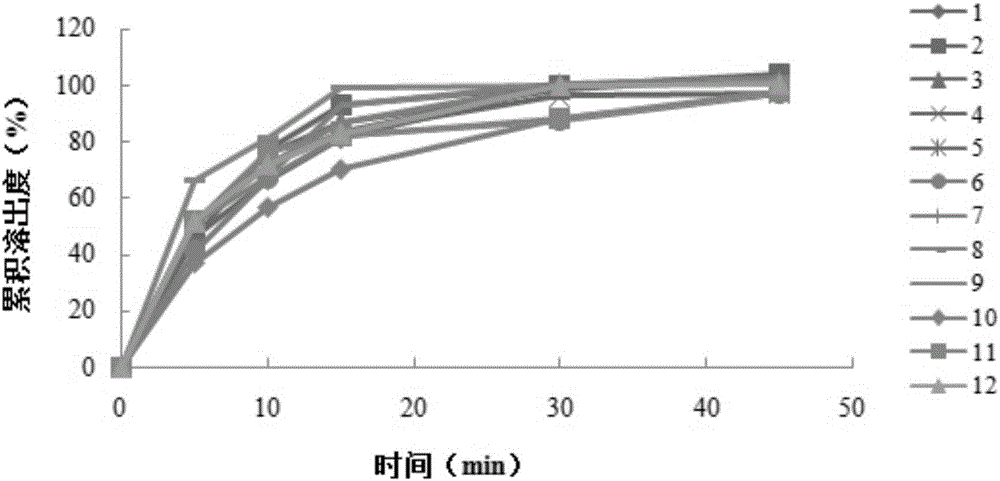
Embodiment 1
[0040] An aluminum-magnesium-pirin tablet (II), made of an aspirin layer and a buffer laminate, wherein the aspirin layer is made of the following raw materials: 81g of aspirin, 40.88g of mannitol, 10g of cornstarch, 0.85g of tartaric acid, Gelatin 0.12g, lemon yellow aluminum lake 0.05g, silicon dioxide 1.1g; The buffer layer is made of the following raw materials: aluminum glycolate 11g, heavy magnesium carbonate 22g, mannitol 30g, cornstarch 29.85g, Gelatin 0.15g, silicon dioxide 1g.
[0041] The preparation technology of this aluminum-magnesium-pirin tablet (II) adopts the following steps:
[0042] (1) get above-mentioned raw material aspirin of aspirin layer, tartaric acid and mannitol, cross 80 mesh sieves after being pulverized respectively; Get above-mentioned raw material cornstarch of aspirin layer, cross 80 mesh sieves, get aspirin powder, tartaric acid powder, mannitol powder and cornstarch A ,spare;
[0043] (2) get the gelatin of above-mentioned weight of aspir...
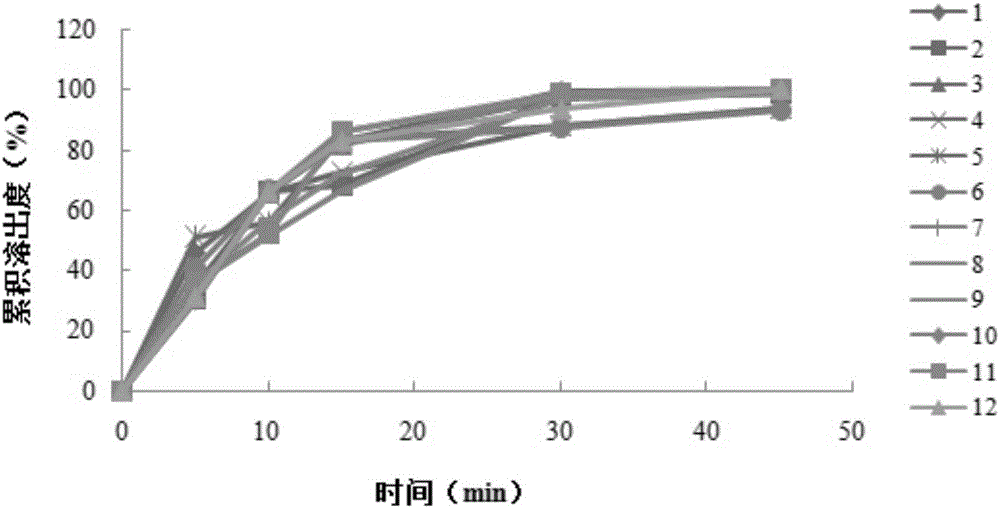
Embodiment 2
[0054] An aluminum-magnesium-pirin tablet (II), made of an aspirin layer and a buffer laminate, wherein the aspirin layer is made of the following raw materials: 81 g of aspirin, 38 g of mannitol, 8 g of cornstarch, 0.6 g of tartaric acid, and gelatin 0.1g, tartrazine aluminum lake 0.03g, silicon dioxide 0.9g; The buffer layer is made of the following raw materials: aluminum glycolate 11mg, heavy magnesium carbonate 22g, mannitol 28g, cornstarch 27g, gelatin 0.12 g, silicon dioxide 0.8g.
[0055] The preparation technology of this aluminum-magnesium-pirin tablet (II) adopts the following steps:
[0056] (1) get above-mentioned raw material aspirin of aspirin layer, tartaric acid and mannitol, cross 80 mesh sieves after being pulverized respectively; Get above-mentioned raw material cornstarch of aspirin layer, cross 80 mesh sieves, get aspirin powder, tartaric acid powder, mannitol powder and cornstarch A ,spare;
[0057] (2) Get the gelatin of the above-mentioned weight of ...
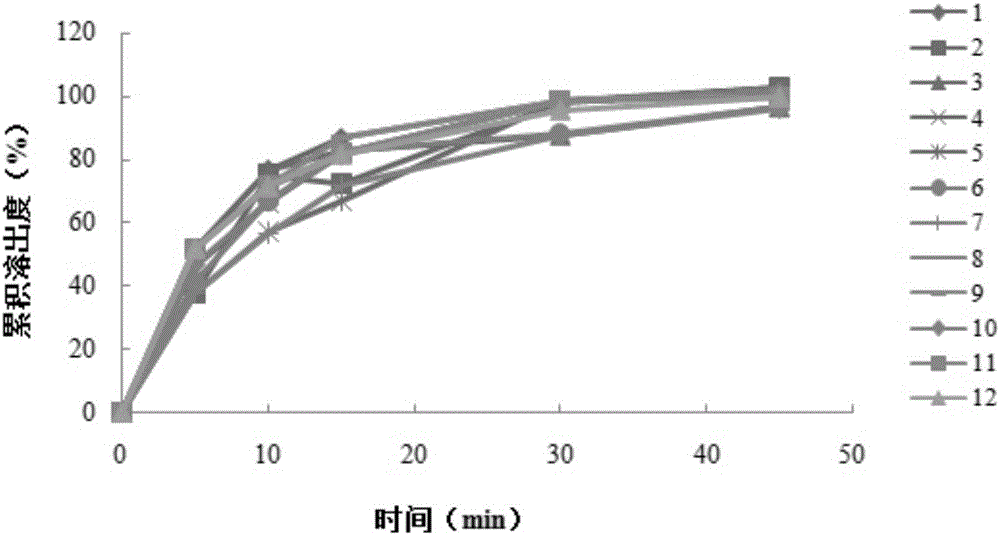
Embodiment 3
[0068] An aluminum-magnesium-pirin tablet (II), made of an aspirin layer and a buffer laminate, wherein the aspirin layer is made of the following raw materials: 81 g of aspirin, 42 g of mannitol, 12 g of cornstarch, 1.0 g of tartaric acid, and gelatin 0.14g, lemon yellow aluminum lake 0.07g, silicon dioxide 1.3g; The buffer layer is made of the following raw materials: aluminum glycolate 11g, heavy magnesium carbonate 22g, mannitol 32g, cornstarch 31g, gelatin 0.18 g, silicon dioxide 1.2g.
[0069] The preparation technology of this aluminum-magnesium-pirin tablet (II) adopts the following steps:
[0070] (1) get above-mentioned raw material aspirin of aspirin layer, tartaric acid and mannitol, cross 80 mesh sieves after being pulverized respectively; Get above-mentioned raw material cornstarch of aspirin layer, cross 80 mesh sieves, get aspirin powder, tartaric acid powder, mannitol powder and cornstarch A ,spare;
[0071] (2) get the gelatin of above-mentioned weight of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com