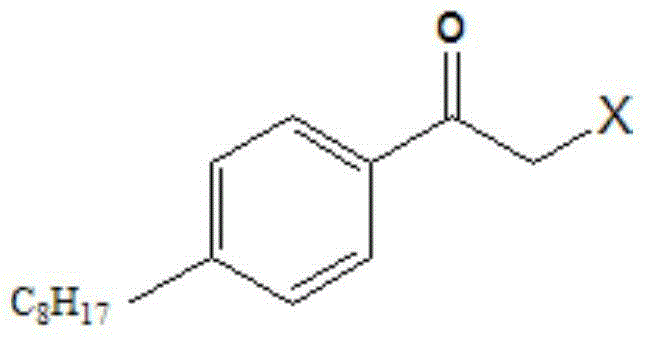
Preparation method and preparation intermediate of fingolimod hydrochloride
A technology for fingolimod hydrochloride and an intermediate, which is applied in the field of pharmaceutical synthesis, can solve the problems of column purification, complicated operation, low yield and the like, and achieves the effects of easy industrial production, advanced preparation technology and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] step 1
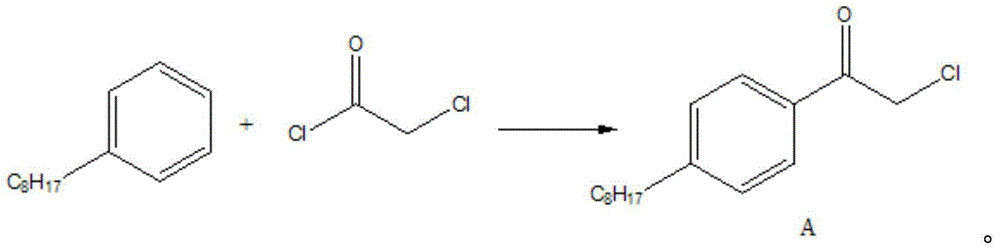
[0034] In a 250mL three-necked flask, add 19g (0.1mol) of n-octylbenzene, 13.3g (0.1mol) of aluminum trichloride, and 35mL of dichloromethane, cool to -10°C, add dropwise 11.3g (0.1mol) of chloroacetyl chloride in 30mL of dichloromethane solution, the dropwise addition temperature is between 0°C, after the dropwise addition is completed, stir at room temperature for 2 hours. Pour the reaction solution into 200 mL of ice water with stirring, separate the organic phase, extract the water phase with 50 mL of dichloromethane, combine the organic phases, wash twice with water, dry over anhydrous magnesium sulfate, filter and concentrate, add 30 mL of petroleum ether to the residue , placed in the refrigerator overnight, and the precipitated solid was suction-filtered to obtain 17 g of a white solid (Compound A).
[0035] step 2
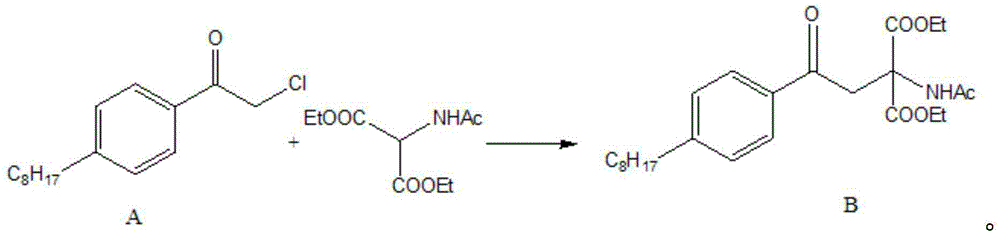
[0036] In a 500mL three-neck flask, add 200mL of absolute ethanol, cut 4.6 (0.2mol) g of sodium metal into small pieces and slowly add t...
Embodiment 2
[0049] step 1
[0050] In a 250mL three-necked flask, add 19g (0.1mol) of n-octylbenzene, 13.3g (0.1mol) of aluminum trichloride, and 35mL of dichloromethane, cool to -10°C, add dropwise 11.3g (0.1mol) of chloroacetyl chloride in 30mL of dichloromethane solution, the dropwise addition temperature is between -5-0°C, after the dropwise addition is completed, stir at room temperature for 2 hours. Pour the reaction solution into 200 mL of ice water with stirring, separate the organic phase, extract the water phase with 50 mL of dichloromethane, combine the organic phases, wash twice with water, dry over anhydrous magnesium sulfate, filter and concentrate, add 30 mL of petroleum ether to the residue , placed in the refrigerator overnight, and the precipitated solid was suction-filtered to obtain 17.2 g of a white solid (Compound A).
[0051] step 2
[0052] In a 500mL three-neck flask, add 200mL of absolute ethanol, cut 4.6 (0.2mol) g of sodium metal into small pieces and slowly ...
Embodiment 3
[0065] step 1
[0066] In a 250mL three-necked flask, add 19g (0.1mol) of n-octylbenzene, 13.3g (0.1mol) of aluminum trichloride, and 35mL of dichloromethane, cool to -10°C, add dropwise 11.3g (0.1mol) of chloroacetyl chloride in For a solution of 30 mL of dichloromethane, the dropwise addition temperature is between -10°C and 5°C. After the dropwise addition is complete, stir at room temperature for 2 hours. Pour the reaction solution into 200 mL of ice water with stirring, separate the organic phase, extract the water phase with 50 mL of dichloromethane, combine the organic phases, wash twice with water, dry over anhydrous magnesium sulfate, filter and concentrate, add 30 mL of petroleum ether to the residue , placed in the refrigerator overnight, and the precipitated solid was suction-filtered to obtain 17.3 g of a white solid (Compound A).
[0067] step 2
[0068] Add 200mL of absolute ethanol to a 500mL three-necked flask, cut 4.6 (0.2mol) g of sodium metal into small p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com