Method for preparing compound aspirin double-layer tablet
A double-layer tablet, aluminum-magnesium technology, applied in the direction of aluminum/calcium/magnesium active ingredients, pharmaceutical formulations, medical preparations with non-active ingredients, etc., can solve the problems of easy decomposition, affecting the stability of aspirin, etc. Dissolution effect is good, the effect of avoiding irritation and damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
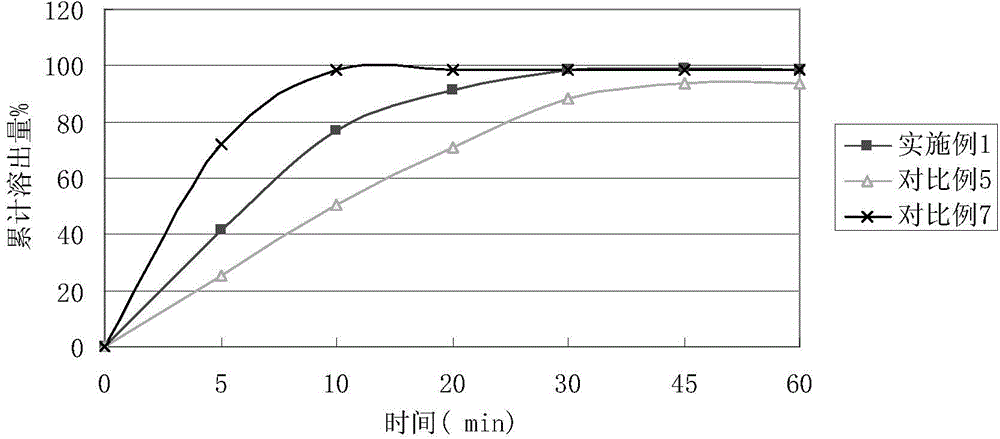
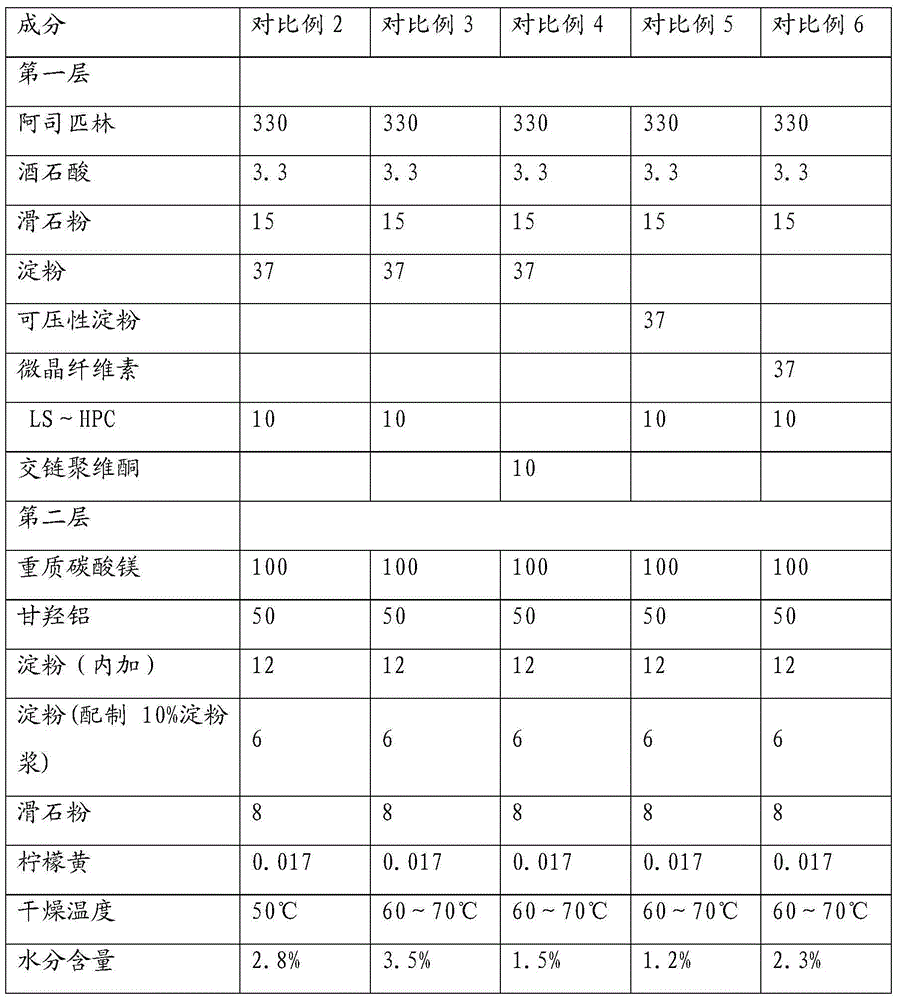
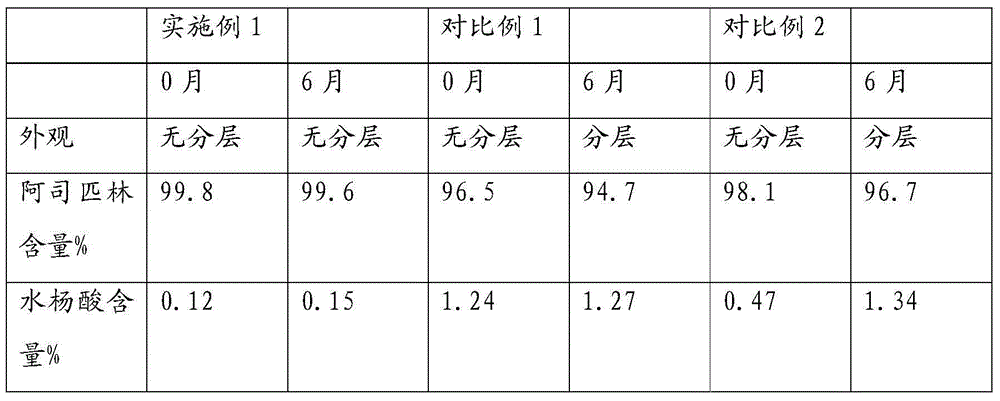
Image
Examples
Embodiment 1
[0026] The preparation of Al-Mg double-layer tablets is based on the amount of 1000 tablets, and the preparation process is as follows:
[0027] 1) Mix 330g of aspirin through a 100-mesh sieve, 3.3g of tartaric acid, 10g of low-substituted hydroxypropyl cellulose, 37g of starch and 15g of talcum powder, and put them into a tablet machine to compress to obtain tablet A;
[0028] 2) After dissolving 0.017g of lemon yellow with a small amount of purified water, add 6g of starch, then add a small amount of purified water and stir evenly, then add boiling purified water, continue stirring until the slurry is cooked, and prepare 10% starch slurry;
[0029] 3) Grind 100g of heavy magnesium carbonate and 50g of glyoxate, pass through a 40-mesh sieve, mix with 12g of starch evenly, add a small amount of starch slurry in step 2) and stir at high speed for 200 seconds, then gradually pour in the remaining starch slurry and stir at high speed 1. Cut for 60 seconds, granulate, pass through...
Embodiment 2
[0033] The preparation of Al-Mg double-layer tablets is based on the amount of 1000 tablets, and the preparation process is as follows:
[0034] 1) Mix 330g of aspirin through a 100-mesh sieve, 3.3g of tartaric acid, 8g of low-substituted hydroxypropyl cellulose, 40g of starch and 12g of talcum powder, and put them into a tablet machine to compress to obtain tablet A;
[0035] 2) After dissolving 0.015 g of lemon yellow with a small amount of purified water, add 4 g of starch, then add a small amount of purified water and stir evenly, then add boiling purified water, continue stirring until the slurry is cooked, and prepare 10% starch slurry;
[0036] 3) Grind 100g of heavy magnesium carbonate and 50g of glyoxate, pass through a 40-mesh sieve, mix evenly with 10g of starch, add a small amount of starch slurry from step 2) and stir for 200 seconds at high speed, then gradually pour in the remaining starch slurry and stir at high speed 1. Cut for 60 seconds, granulate, pass thro...
Embodiment 3
[0040] The preparation of Al-Mg double-layer tablets is based on the amount of 1000 tablets, and the preparation process is as follows:
[0041] 1) Mix 330g of aspirin through a 100-mesh sieve, 3.3g of tartaric acid, 15g of low-substituted hydroxypropyl cellulose, 30g of starch and 18g of talcum powder, and put them into a tablet machine to compress into tablets to obtain tablet A;
[0042] 2) After dissolving 0.02 g of lemon yellow with a small amount of purified water, add 8 g of starch, then add a small amount of purified water and stir evenly, then add boiling purified water, continue stirring until the slurry is cooked, and prepare 10% starch slurry;
[0043] 3) Pass 100g of heavy magnesium carbonate and 50g of aluminum glyoxate through a 40-mesh sieve, mix evenly with 15g of starch, add the starch slurry in step 2), wet granulate, and pass through a 16-mesh sieve for granulation. Dry at a temperature of ℃, and when the moisture content of the granules is ≤ 3%, stop heating...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com