Biguanide derivative for preventing and treating infarction diseases and application thereof
A biguanide derivative and infarction technology, which is applied in blood diseases, extracellular fluid diseases, cardiovascular system diseases, etc., can solve the problems of lack of mode of action and mechanism, and ineffective effects, so as to reduce production costs and achieve high economic efficiency , simple structure and synthetic process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
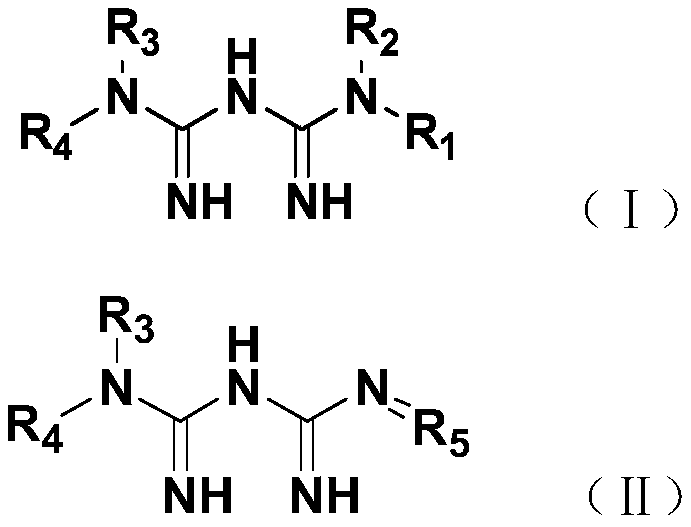
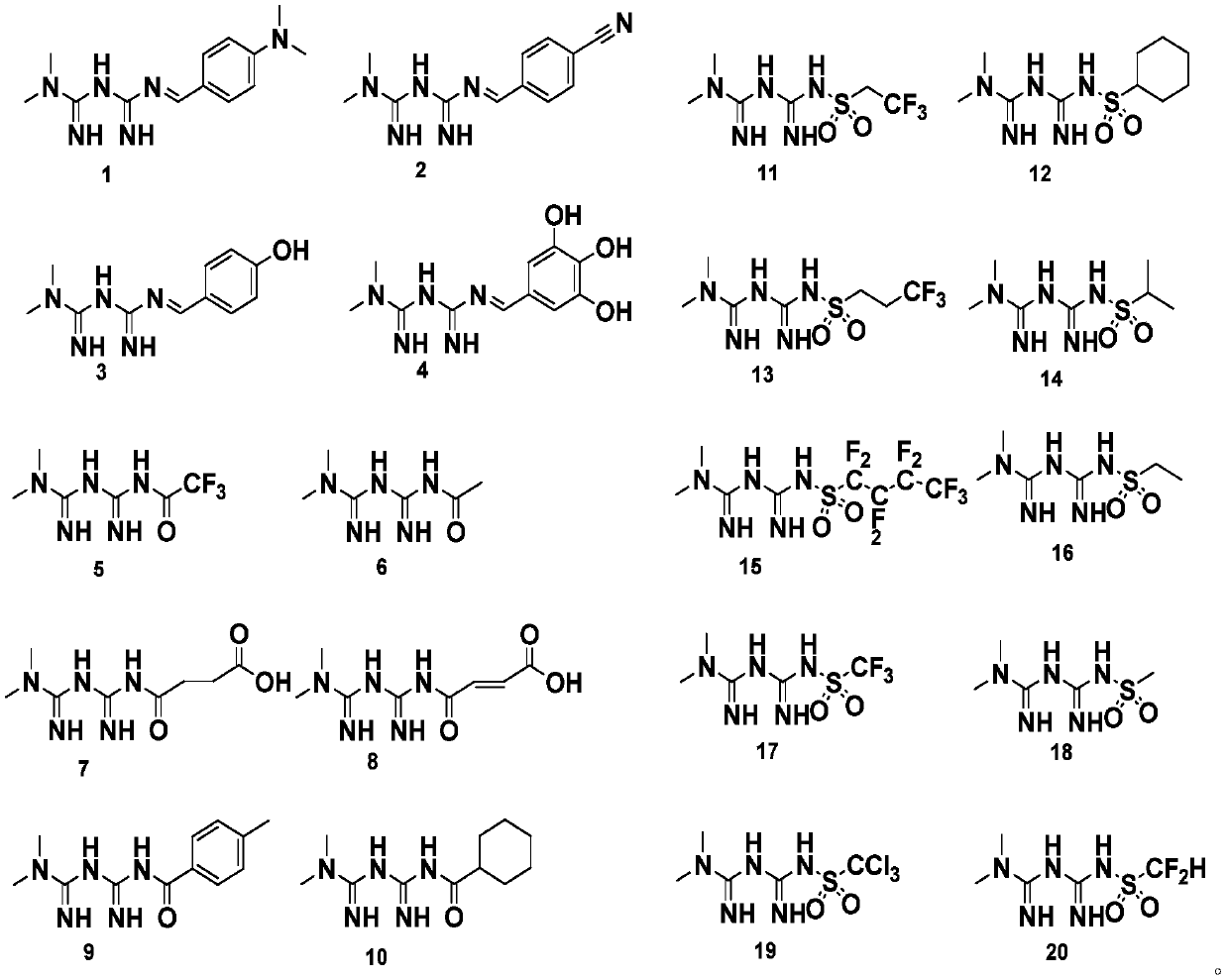
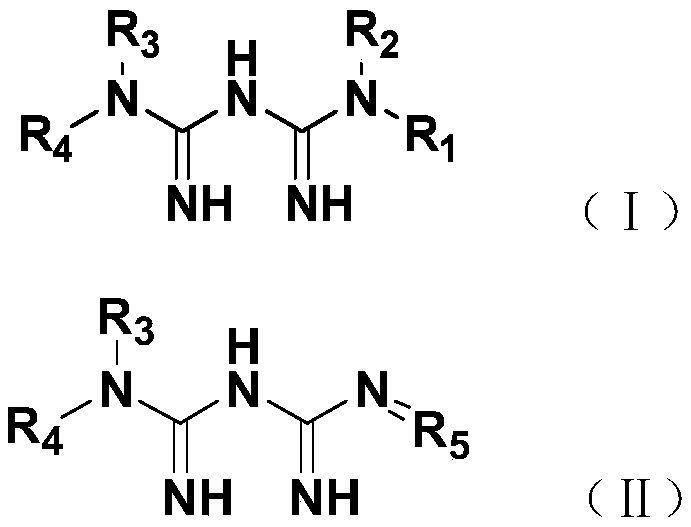
[0023] In this example, guanidinated metformin derivatives were synthesized, specifically including compounds 1-4.
[0024] 1. Synthesis of compound 1: N-(4-dimethylaminobenzylidene) metformin
[0025] Take 1.29g (10.0mmol) of metformin, 1.49g (10.0mmol) of 4-dimethylaminobenzaldehyde, 150mL of ethanol, and 2mL of acetic acid in a 250mL flask, and carry out a stirring reaction at 78°C. The progress of the reaction was detected by TCL, and the reaction was basically complete after about 6 hours. Add sodium bicarbonate to adjust the pH value to neutral to quench the reaction, then spin dry under reduced pressure to obtain a crude product, and then use petroleum ether: ethyl acetate = 2:1 (v / v) as an eluent to pass through a silica gel column, 2.3 g of compound 1 was obtained as a white powder with a yield of 90%. R f is 0.6 (dichloromethane:methanol:glacial acetic acid=9:1:0.025).
[0026] Compound 1 was detected by proton NMR spectrum, carbon NMR spectrum and mass spectrome...
Embodiment 2
[0045] In this example, sulfonamide metformin derivatives were synthesized, specifically including compounds 11-20.
[0046] 1. Synthesis of compound 11: N-(2,2,2-trifluoroethylsulfonyl) metformin
[0047] Take 1.29g (10.0mmol) metformin, 1.82g (10.0mmol) 2,2,2-trifluoroethylsulfonyl chloride, 300mL anhydrous acetone, 0.056g (1mmol) potassium hydroxide in a 500mL flask, and stir overnight at room temperature. Use TCL to detect the reaction process. When there is no obvious change in the reaction product, add dilute hydrochloric acid to adjust the pH value to neutral to quench the reaction, then spin dry under reduced pressure to obtain the crude product, and then use petroleum ether:ethyl acetate=2:1 (v / v) was used as an eluent to pass through a silica gel column to obtain 2.5 g of compound 11 as a white powder, with a yield of 90%. R f is 0.7 (dichloromethane:methanol:glacial acetic acid=9:1:0.025).
[0048] Compound 11 was detected by H NMR spectrum, C NMR spectrum and ma...
Embodiment 3
[0097] In this example, amidated metformin derivatives were synthesized, specifically including compounds 5-10.
[0098] 1. Synthesis of compound 5: N-(2,2,2-trifluoroacetyl) metformin
[0099] Take 1.29g (10.0mmol) metformin, 2.10g (10.0mmol) trifluoroacetic anhydride, 300mL anhydrous acetone, 0.056g (1mmol) potassium hydroxide in a 500mL flask, and stir overnight at room temperature. Use TCL to detect the reaction process. When there is no obvious change in the reaction product, add dilute hydrochloric acid to adjust the pH value to neutral to quench the reaction, then spin dry under reduced pressure to obtain the crude product, and then use petroleum ether:ethyl acetate=2:1 (v / v) was used as an eluent to pass through a silica gel column to obtain 2.0 g of white powder compound 5 with a yield of 89%. R f is 0.7 (dichloromethane:methanol:glacial acetic acid=9:1:0.025).
[0100] Compound 5 was detected by proton nuclear magnetic spectrum, carbon nuclear magnetic spectrum an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com