Azithromycin derivative and its use
A technology of azithromycin and derivatives, applied in the field of azithromycin derivatives, can solve the problems of poor activity of macrolide-resistant bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
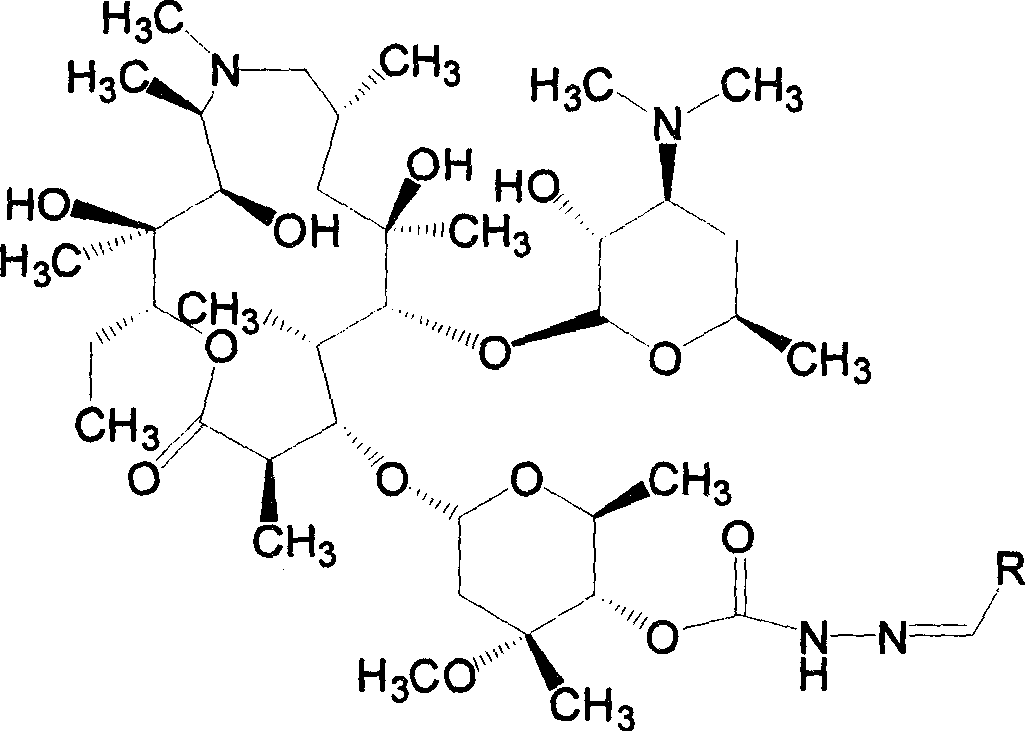
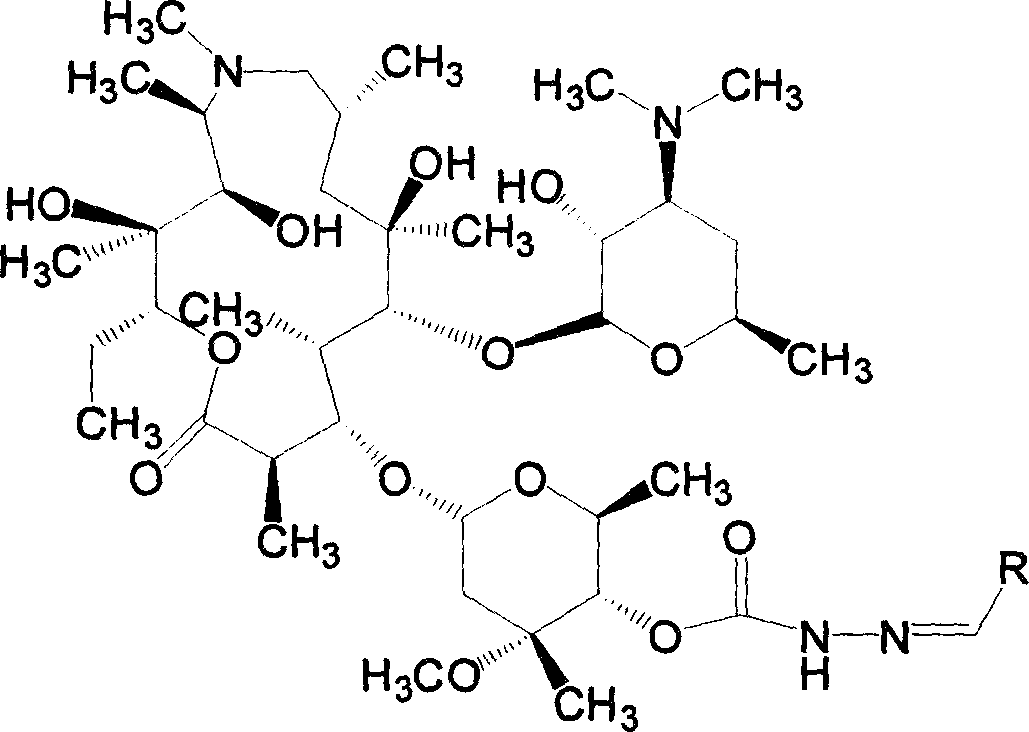
[0061] Example 1 4"-(2-benzylidene) carbazate azithromycin (compound 1)
[0062]Synthesize 4"-carbazate azithromycin (F) (0.620mmol), benzaldehyde (1.240mmol) and acetic acid (1.860mmol) according to the general method, then evaporate the solvent, add ethyl acetate to dissolve, wash with water, and anhydrous Dry over sodium sulfate, filter, evaporate the solvent to dryness under reduced pressure, dry column chromatography, successively use ethyl acetate:petroleum ether:diethylamine volume ratio as 3:5:0.5 and 5:5:1 as eluent, 0.476 g of the product was obtained, and the yield was 85.8%.
[0063] MS(ESI+):895[M+H] + . 1 HNMR (400M, CDCl 3 )δ 8.3 (1H, s), 7.9 (1H, s), 7.7 (2H, m), 7.4 (3H, m), 5.2 (1H, d, 11-CH-), 4.7 (1H, d), 4.5 (1H, d), 4.3 (1H, m), 3.35 (3H, s, 3"-OCH 3 ), 2.25(6H, s, 3'-N(CH 3 ) 2 ).
Embodiment 2
[0064] Example 2 4"-[2-(2-phenylvinylmethylene)]carbazate azithromycin (compound 2)
[0065] Synthesize 4"-carbazate azithromycin (F) (0.620mmol), cinnamaldehyde (1.240mmol) and acetic acid (1.860mmol) according to the general method, evaporate the solvent, add ethyl acetate to dissolve, wash with water, anhydrous sulfuric acid Sodium drying, filtration, evaporated to dryness under reduced pressure, dry column chromatography, the volume ratio of ethyl acetate:petroleum ether:diethylamine is 3:4:0.5 as eluent, and the product is 0.098g, yield 17.2% .
[0066] MS(ESI+):921[M+H] + . 1 HNMR (400M, CDCl 3 )δ 8.9 (1H, s), 7.8 (1H, s), 7.3 (5H, m), 6.9 (1H, s), 6.8 (1H, s), 5.2 (1H, d, 11-CH-), 4.7 (1H,d), 4.5(1H,d), 4.3(1H,m), 3.35(3H,s,3"-OCH 3 ), 2.35(6H, s, 3'-N(CH 3 ) 2 ).
[0067] 13 CNMR (400M, CDCl 3 )δ 178.5, 148.0, 138.7, 135.8, 128.8, 128.8, 128.7, 126.8, 125.0, 102.6, 95.0, 83.9, 79.9, 78.5, 77.4, 77.3, 77.0, 76.7, 74.2, 73.5, 73.1, 79.06, 67. 63.2, 62.3, 60.3,...
Embodiment 3
[0068] Example 3 4"-[2-(4-methoxybenzylidene)]carbazate azithromycin (compound 3)
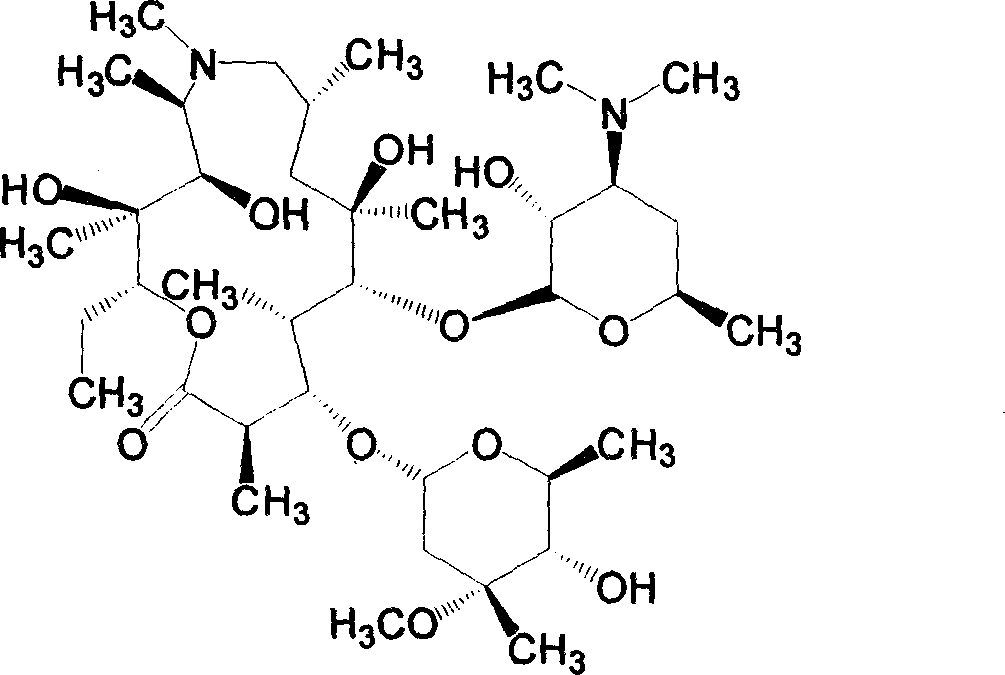
[0069] Synthesize 4"-carbazate azithromycin (F) (0.620mmol), p-methoxybenzaldehyde (1.240mmol) and acetic acid (1.860mmol) according to the general method, evaporate the solvent, add ethyl acetate to dissolve, wash with water , dried over anhydrous sodium sulfate, filtered, evaporated to dryness under reduced pressure, dry column chromatography, ethyl acetate: petroleum ether: diethylamine volume ratio is 3: 5: 0.5 as eluent, to obtain yellowish foam Add ethyl acetate to dissolve, add water to stir, cool in an ice-water bath, adjust the pH of the solution to 3 with 1.0N hydrochloric acid, and separate the water phase. Under the condition of continued cooling in the ice-water bath, adjust the pH to 9 with 3.0N sodium hydroxide solution , extracted with ethyl acetate, combined the organic phases, washed with water, anhydrous Na 2 SO 4 After drying, filtering, and evaporating the solvent under r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com