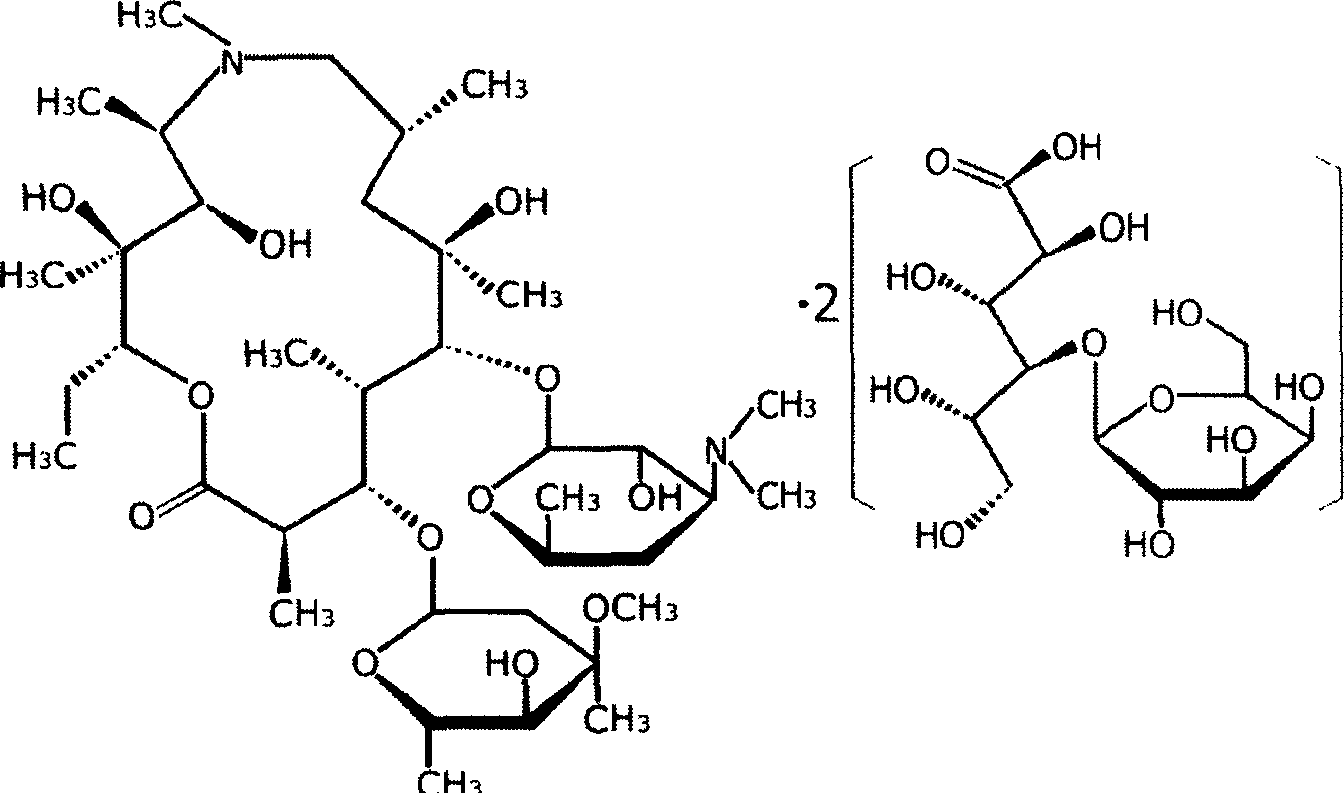
Eye preparation containing lactose-azithromycin
A technology of azithromycin and lactobionic acid, which is applied in the field of pharmaceuticals, can solve the problems of loss of antibacterial activity, uneven drug dosage, eye irritation, etc., and achieve the effect of reducing re-pollution, no appearance and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Consists of the following components and mass percentages, which contain: azithromycin lactobionate 0.59% (as azithromycin 0.3%), sodium bisulfite 1.0%, povidone K 30 0.5%, hydrogenated castor oil 0.8%, medium chain glycerides 0.1%, Transcutol-p 0.4%, boric acid 1.0%, borax 0.2%, lactose 0.75%, ethyl paraben 0.03%, and the rest is water. The preparation process is the same as above.
Embodiment 2
[0041] Example 2: It is composed of the following components and mass percentages, which contains: 0.59% azithromycin lactobionate (0.3% as azithromycin), 0.6% vitamin C, and povidone K 30 0.5%, hydrogenated castor oil 0.8%, medium chain glycerides 0.1%, Transcutol-p 0.4%, boric acid 1.0%, borax 0.2%, lactose 0.75%, ethyl paraben 0.03%, and the rest is water. The preparation process is the same as above.
Embodiment 3
[0042] Example 3: Consists of the following components and mass percentages, which contain: azithromycin lactobionate 0.59% (0.3% as azithromycin), carbopol 0.5%, borax 1.4%, sodium bisulfite 1.0%, hydrogenated castor oil 1.5%, 0.3% medium chain glycerides, 0.6% Transcutol-p, 0.1% boric acid, 0.5% lactose, 0.03% ethyl paraben, and the rest is water. The preparation process is the same as above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com