Azithromycin sustained release tablets and method of preparing the same
A technology of azithromycin and sustained-release tablets, which is applied in the field of medicine to achieve the effects of improving compliance and convenience, reducing drug resistance, and taking convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Component weight / 1000 pieces
[0020] Azithromycin 500g
[0021] Hydroxypropyl Methyl Cellulose 140g
[0022] Lactose 220g
[0023] Microcrystalline Cellulose 130g
[0025] 90% ethanol appropriate amount
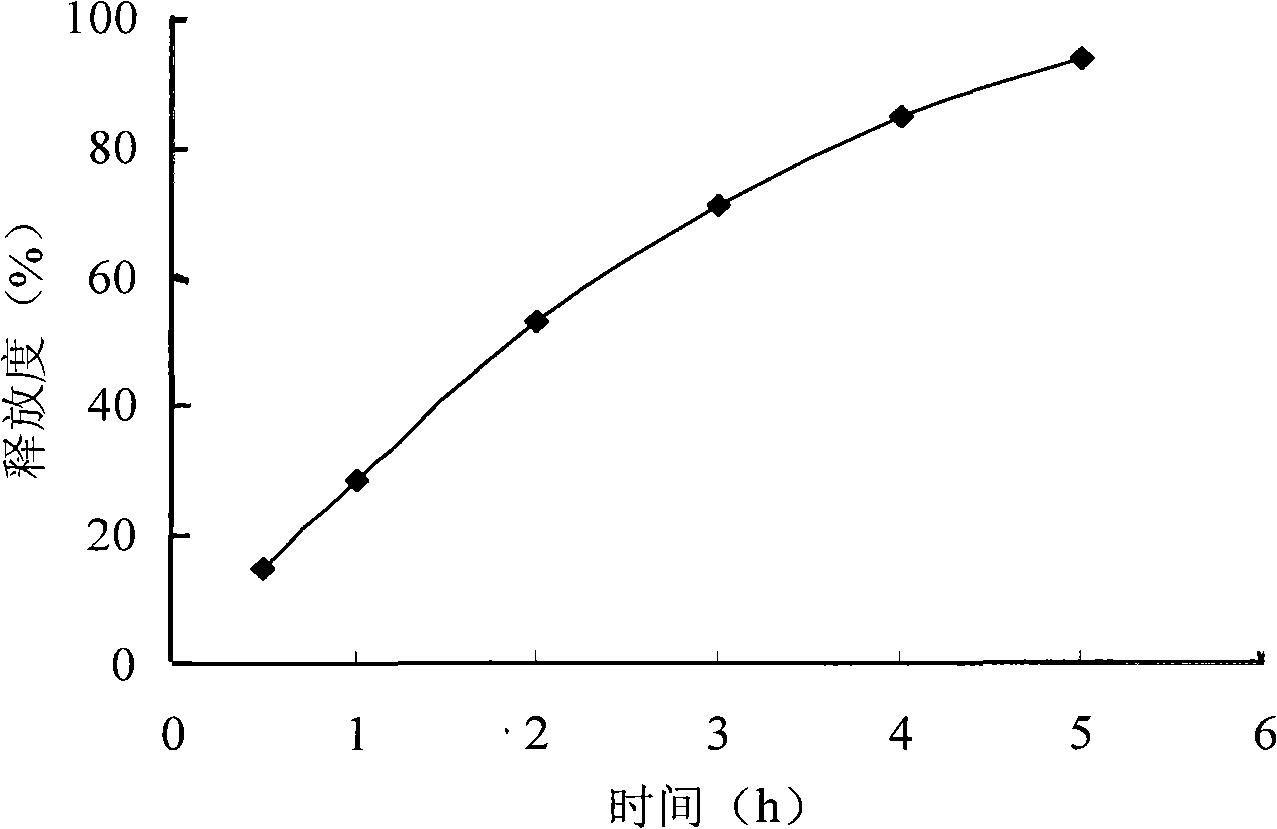
[0026] Grind the ingredients and excipients of the prescription separately and pass through a 100-mesh sieve for later use; mix azithromycin, hydroxypropyl methylcellulose, microcrystalline cellulose and lactose through a 100-mesh sieve, and use an appropriate amount of 90% ethanol to make a soft material, 20 mesh Sieve to granulate, dry the wet granules at 60±5°C, sieve and granulate with 20 meshes; add magnesium stearate, mix well, and tablet. Release curve see figure 1 .
Embodiment 2
[0028] Component Weight
[0029] / 1000 pieces
[0030] Azithromycin 500g
[0031] Polyoxyethylene 200g
[0032] Hydroxypropyl Cortyl Cellulose 150g
[0033] Microcrystalline Cellulose 140g
[0035] 90% ethanol appropriate amount
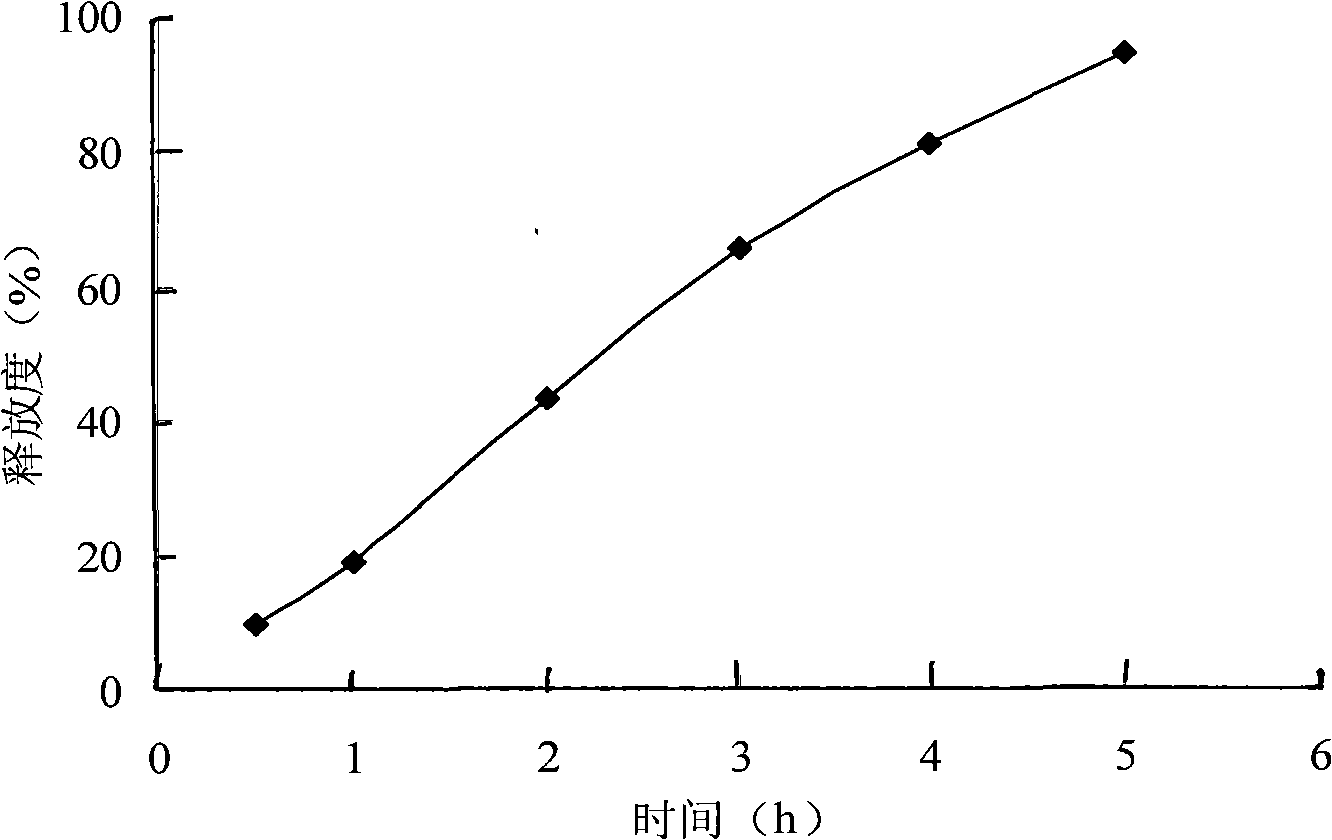
[0036] Grind the raw materials and auxiliary materials of the prescription separately and pass them through a 100-mesh sieve for later use; weigh the prescribed amount of azithromycin, hydroxypropyl methylcellulose, polyoxyethylene and microcrystalline cellulose and pass through a 100-mesh sieve to mix them evenly. It is a soft material made of adhesive, granulated with a 20-mesh sieve, dried at 60±5°C, granulated with a 20-mesh sieve; added magnesium stearate, mixed evenly, and compressed into tablets. Release curve see figure 2 .
Embodiment 3
[0038] Component Weight
[0039] / 1000 pieces
[0040] Azithromycin 500g
[0041] Sodium Alginate 250g
[0042] Lactose 150g
[0043] Microcrystalline Cellulose 90g
[0045] 90% ethanol appropriate amount
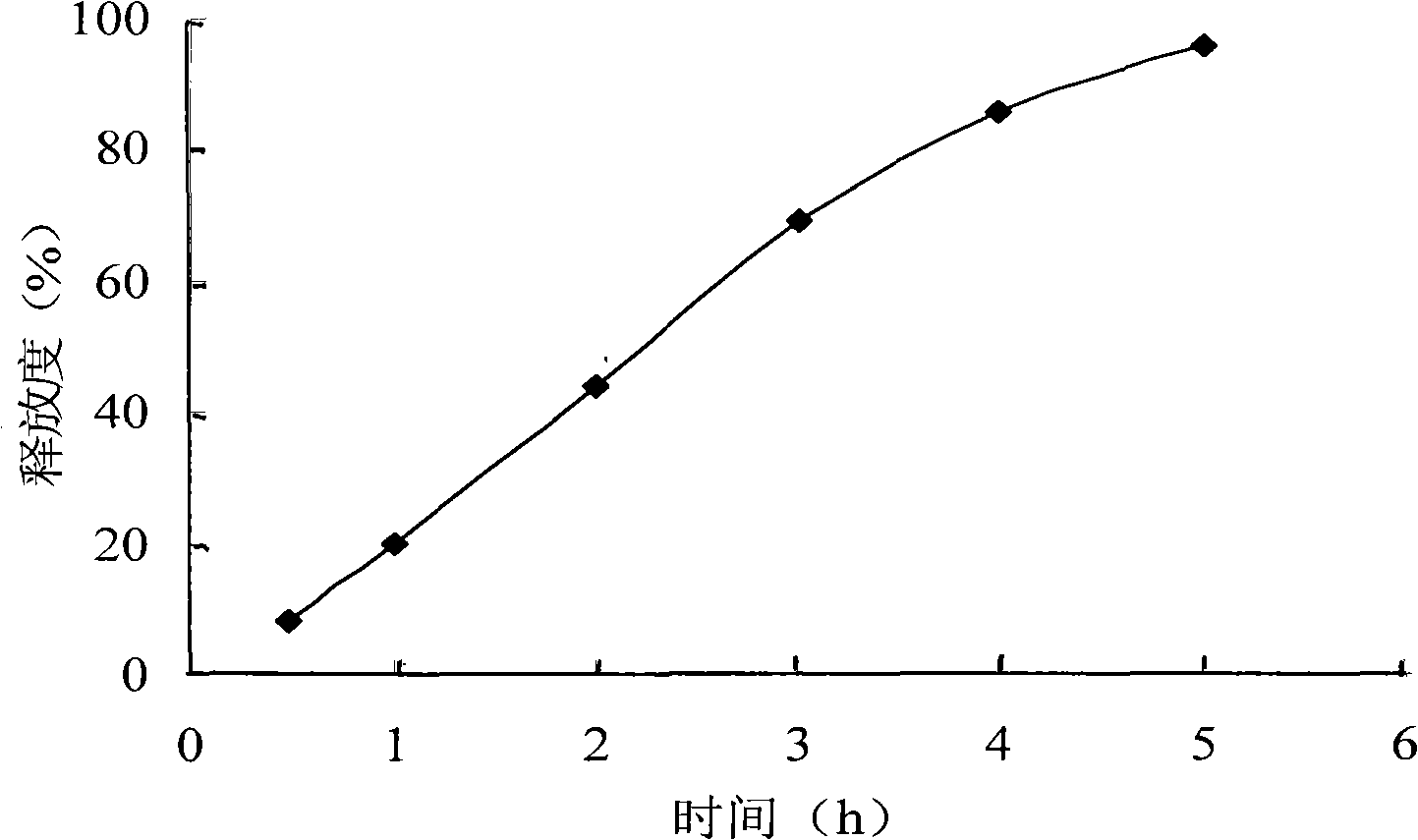
[0046]Grind the raw materials and auxiliary materials of the prescription separately and pass through a 100-mesh sieve for later use; weigh the prescribed amount of azithromycin, sodium alginate, lactose and microcrystalline cellulose and mix them evenly through a 100-mesh sieve, and use an appropriate amount of 90% ethanol as a binder to make a soft material , 20-mesh sieve, granulated, wet granules were dried at 60±5°C, granulated with 20-mesh sieve; magnesium stearate was added, mixed evenly, and tableted. Release curve see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com