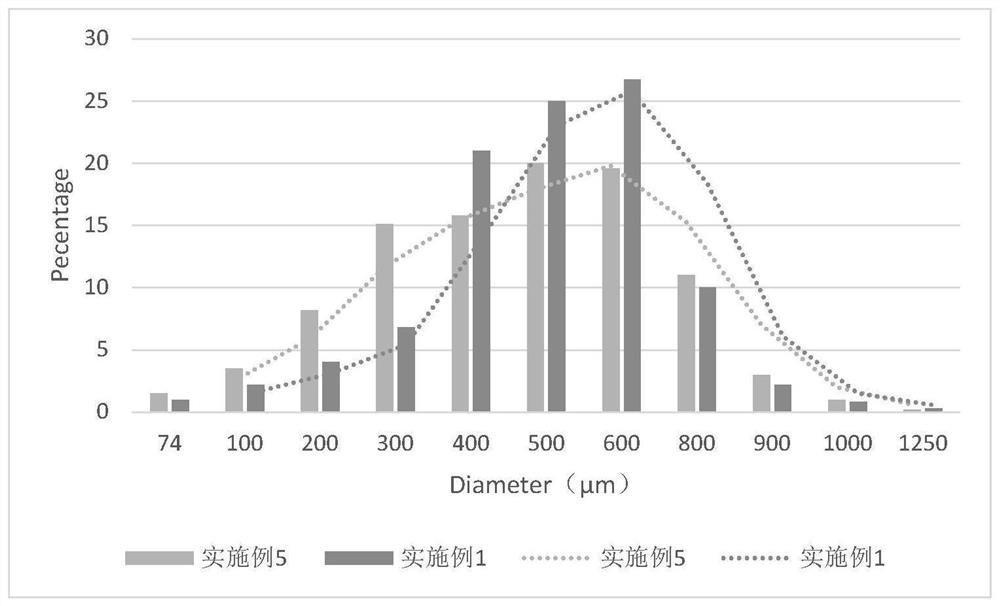
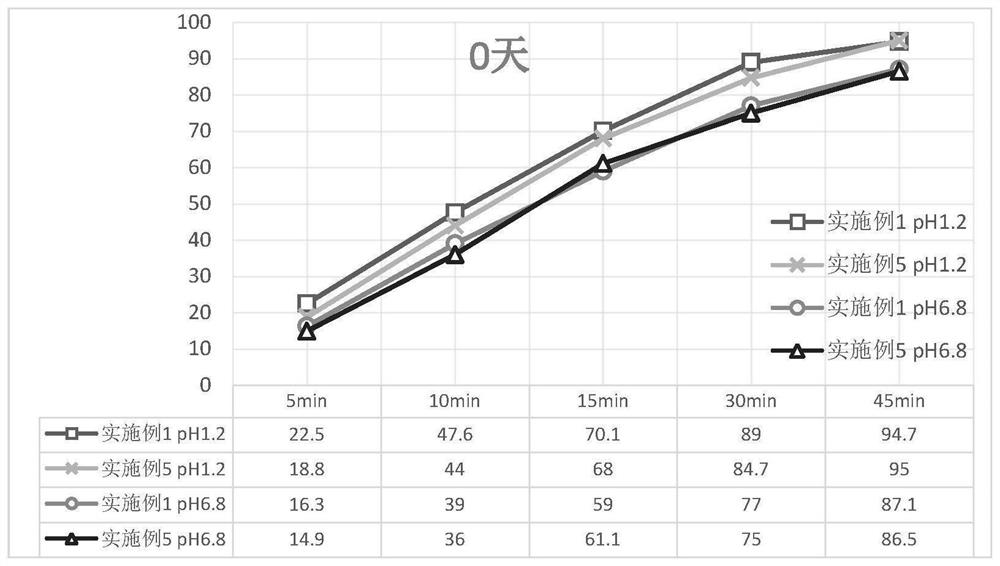
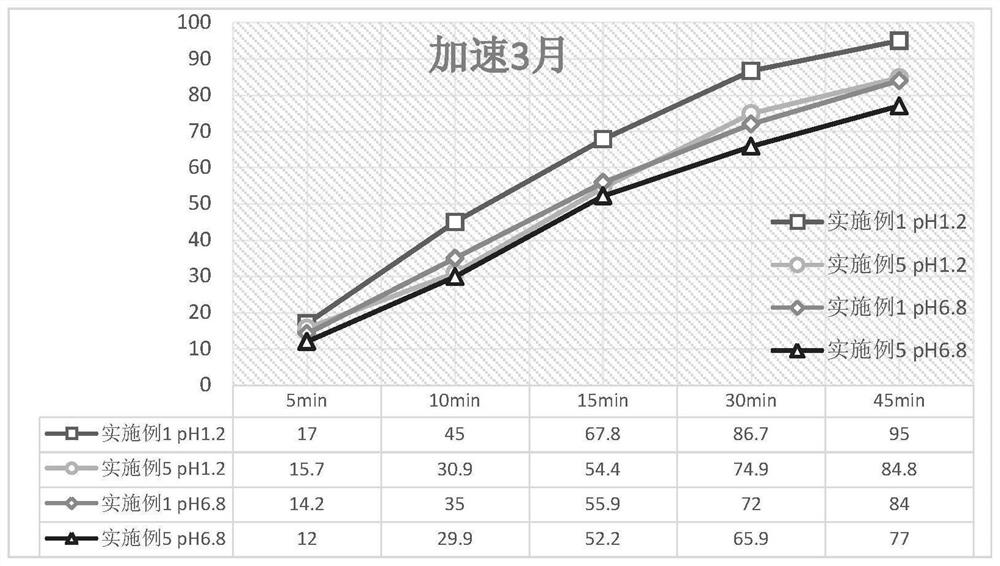
Avatrombopag maleate preparation composition as well as tablet or capsule prepared from composition and preparation method
A technology of alvatrombopag and composition, which is applied in capsule delivery, drug combination, pill delivery, etc. It can solve the problems of decreased dissolution rate, poor water solubility of active ingredients, decreased, accelerated investigation, etc., and achieves good uniformity and API Uniform particle size distribution and high dissolution stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] A stable alvatrombopag maleate tablet with a specification of 20mg / tablet per 1000 tablets prescription:
[0068] Name of raw material Prescription amount Alvatrombopag Maleate (calculated as Alvatrombopag) 20g lactose monohydrate 100g microcrystalline cellulose 60g Crospovidone 10g Hypromellose 4g Magnesium stearate 2g Micropowder silica gel 4g
[0069] Preparation Process:
[0070] a. Premix 20g of alvatrombopag maleate with 80g of lactose and 20g of microcrystalline cellulose, and grind together in a ball mill for 200 minutes;
[0071] b. Add 20g lactose, 40g microcrystalline cellulose, 4g hypromellose, 10g crospovidone, and 1g magnesium stearate to the co-grinding mixture for 10 minutes, then dry granulate, Number 24;
[0072] c. Add 1g of magnesium stearate and 4g of micropowder silica gel to the prepared granules, and mix in a three-position motion mixer for 10 minutes;
[0073] d. Compress the mixed mat...
Embodiment 2
[0075] A kind of stable alvatrombopag maleate capsules, the prescription composition in every 1000 tablets of 20mg / capsules is as follows:
[0076] Name of raw material Prescription amount Alvatrombopag Maleate (calculated as Alvatrombopag) 20g lactose monohydrate 80g microcrystalline cellulose 80g Crospovidone 10g Hypromellose 4g Magnesium stearate 2g Micropowder silica gel 4g
[0077] a. Premix 20g of alvatrombopag maleate with 80g of lactose and 20g of microcrystalline cellulose, and grind together for 100 minutes in a ball mill;
[0078] b. Then add 60g of microcrystalline cellulose, 4g of hypromellose, 10g of crospovidone, and 1g of magnesium stearate to the co-grind, mix for 10 minutes, then dry granulate, the whole grain size is 24 mesh ;
[0079] c. Add 1g of magnesium stearate and 4g of micropowder silica gel to the prepared granules, and mix in a three-position motion mixer for 10 minutes;
[0080] d. Fill t...
Embodiment 3
[0082] A kind of stable alvatrombopag maleate tablet, the prescription composition in every 1000 tablets of 20mg / tablet is as follows:
[0083]
[0084]
[0085] a. Premix 20g of alvatrombopag maleate with 80g of lactose and 20g of microcrystalline cellulose, and grind together in a ball mill for 200 minutes;
[0086] b. Add 10g lactose, 50g microcrystalline cellulose, 4g hypromellose, 10g crospovidone, and 1g magnesium stearate to the co-grinding mixture for 10 minutes, then dry granulate, granulate Number 24;
[0087] c. Add 1g of magnesium stearate and 4g of micropowder silica gel to the prepared granules, and mix in a three-position motion mixer for 10 minutes;
[0088] d. Compress the mixed materials into tablets at a rate of 200mg / tablet, and control the tablet hardness to 100-160N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com