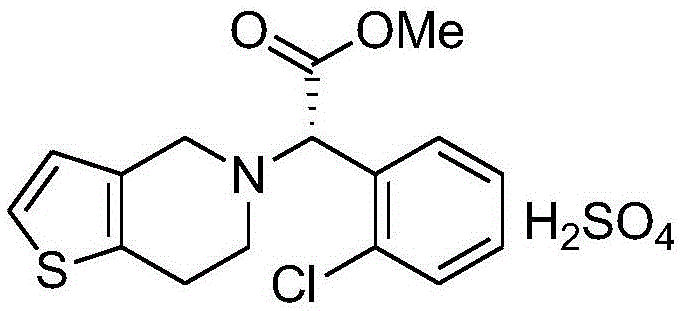
Clopidogrel hydrogen sulfate solid preparation and preparation method thereof
A technology of clopidogrel bisulfate solid and clopidogrel bisulfate, which is applied in the fields of pill delivery, pharmaceutical formula, medical preparations of non-active ingredients, etc., and can solve problems such as too much, difficult to prepare granules, and affecting the dissolution of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
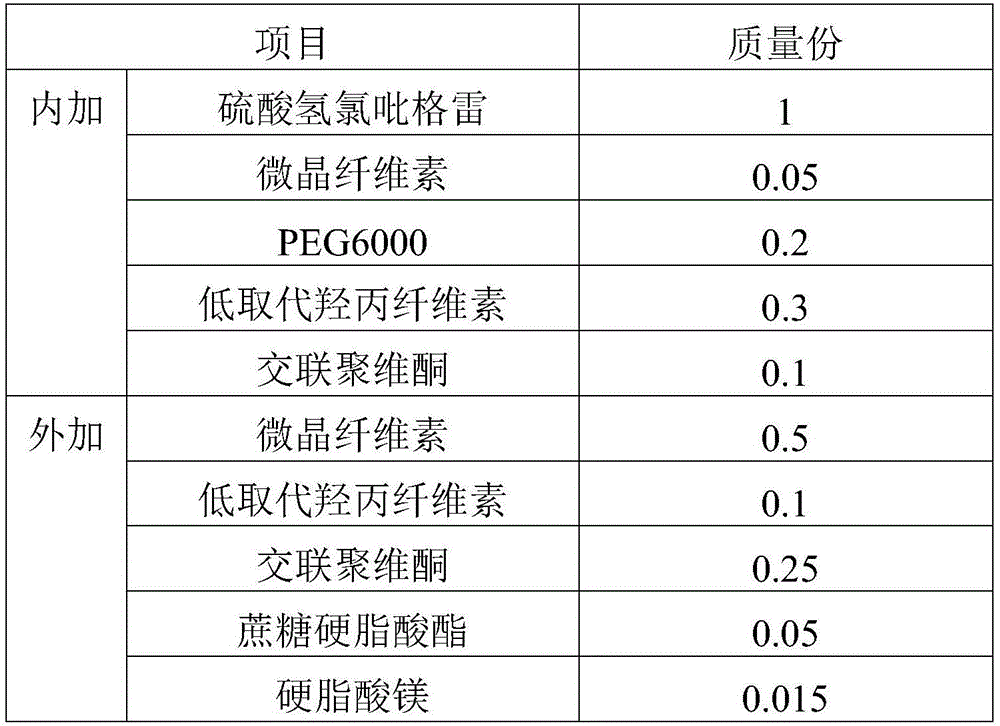
[0067] prescription:
[0068]
[0069]
[0070] Preparation steps:
[0071] 1) Mix the prescribed amount of clopidogrel bisulfate (D90=67 μm) with the internally added excipients for 20-30 minutes to obtain the internally mixed powder;
[0072] 2) Add the internal mixed powder obtained in step 1) into a fluidized bed granulator, and granulate at 50-60°C for 10-30 minutes to obtain internal phase granules (angle of repose 37°). The obtained granules are uniform and almost free of fine powder;
[0073] 3) Mix the introverted granules obtained in step 2) with the prescribed amount of external excipients for 10 to 20 minutes to obtain the total mixed granules;
[0074] 4) Compressing the blended granules obtained in step 3) into tablets to prepare a solid preparation of clopidogrel hydrogen sulfate (75 mg specification).
[0075] No sticking phenomenon occurred in the tableting process, and the obtained clopidogrel bisulfate solid preparation had a smooth surface.
Embodiment 2
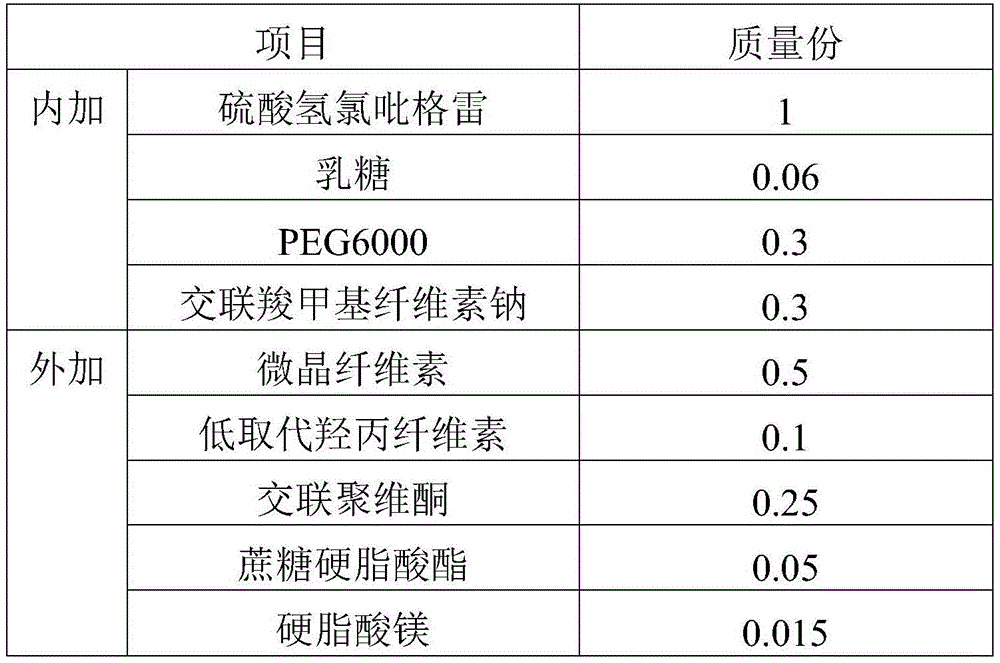
[0077] prescription:
[0078]
[0079] Preparation steps:
[0080] 1) Mix the prescribed amount of clopidogrel bisulfate (D90=60 μm) with the internally added excipients for 20-30 minutes to obtain the internally mixed powder;
[0081] 2) Add the internal mixed powder obtained in step 1) into a fluidized bed granulator, and granulate at 50-60°C for 10-30 minutes to obtain internal phase granules (angle of repose 38°). The obtained granules are uniform and almost free of fine powder;
[0082] 3) Mix the introverted granules obtained in step 2) with the prescribed amount of external excipients for 10 to 20 minutes to obtain the total mixed granules;
[0083] 4) Compressing the blended granules obtained in step 3) into tablets to prepare a solid preparation of clopidogrel hydrogen sulfate (75 mg specification).
[0084] No sticking phenomenon occurred in the tableting process, and the obtained clopidogrel bisulfate solid preparation had a smooth surface.
Embodiment 3
[0086] prescription:
[0087]
[0088] Preparation steps:
[0089] 1) Mix the prescribed amount of clopidogrel hydrogen sulfate (D90=73 μm) with the internally added excipients for 20-30 minutes to obtain the internally mixed powder;
[0090] 2) Add the internal mixed powder obtained in step 1) into a fluidized bed granulator, and granulate at 50-60°C for 10-30 minutes to obtain internal phase granules (angle of repose 38°). The obtained granules are uniform and almost free of fine powder;
[0091] 3) Mix the introverted granules obtained in step 2) with the prescribed amount of external excipients for 10 to 20 minutes to obtain the total mixed granules;
[0092] 4) Compressing the blended granules obtained in step 3) into tablets to prepare a solid preparation of clopidogrel hydrogen sulfate (75 mg specification).
[0093] No sticking phenomenon occurred in the tableting process, and the obtained clopidogrel bisulfate solid preparation had a smooth surface.
PUM
| Property | Measurement | Unit |
|---|---|---|
| D90 | aaaaa | aaaaa |
| D90 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com