Tolterodine Tartrate Film-controlled Sustained-release Pellet Capsules
A technology of tolterod tartrate and sustained-release pellets, which can be used in medical preparations with non-active ingredients, urinary system diseases, organic active ingredients, etc., and can solve problems such as decreased release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The tolterodine tartrate sustained-release pellet capsule of embodiment 1 common ball core
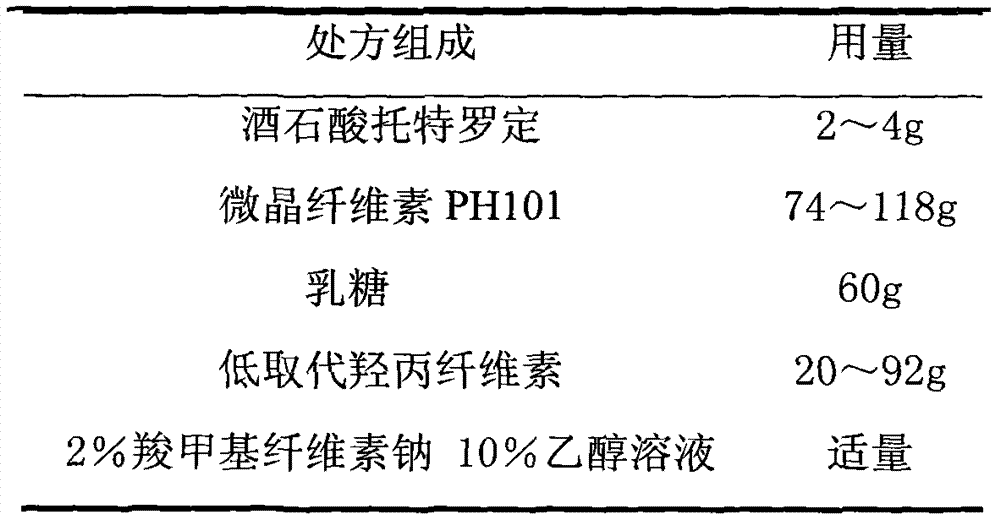
[0028] 1. Prescription
[0029] 1. Pill core prescription (1000 capsules)
[0030]
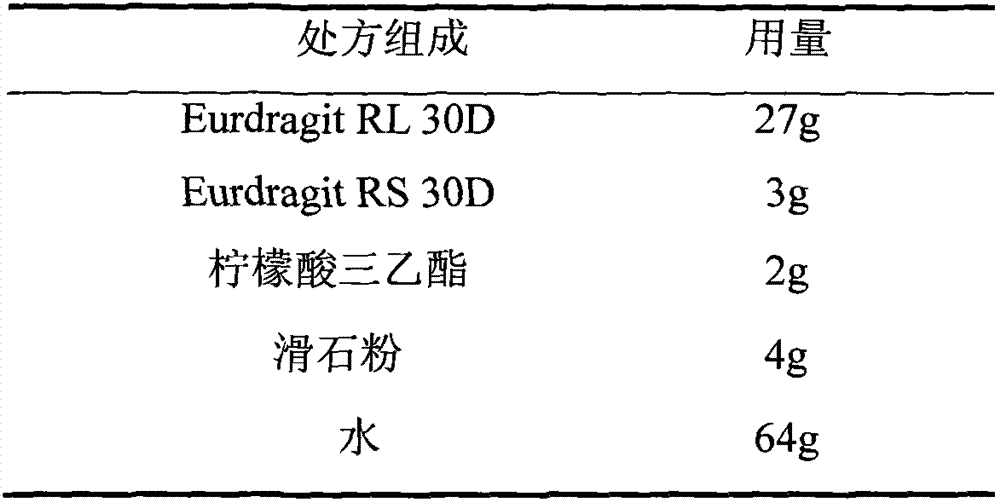
[0031] 2. Prescription of sustained-release film coating solution
[0032]
[0033] 3. No. 0 stomach-soluble gelatin capsule shell 1000 capsules
[0034] Second, the preparation process:
[0035] 1. Ball core preparation process:
[0036] (1) the tolterodine tartrate of prescription quantity is mixed with lactose equal amount progressively;
[0037] (2) Take by weighing the microcrystalline cellulose PH101 of recipe quantity and the tolterodine tartrate and lactose mixture of above-mentioned mixing, put in the wet granulator and mix homogeneously;
[0038] (3) 2% carboxymethylcellulose sodium 10% ethanol solution to make soft materials;
[0039] (4) Extrude on the extruder, the screen aperture is 1.0mm, and the extrusion speed is 20-30rpm;
[0040] (5) spheronization, the spheroniza...
Embodiment 2
[0065] Example 2 Tolterodine tartrate sustained-release pellet capsule containing 10% low-substituted hydroxypropyl cellulose
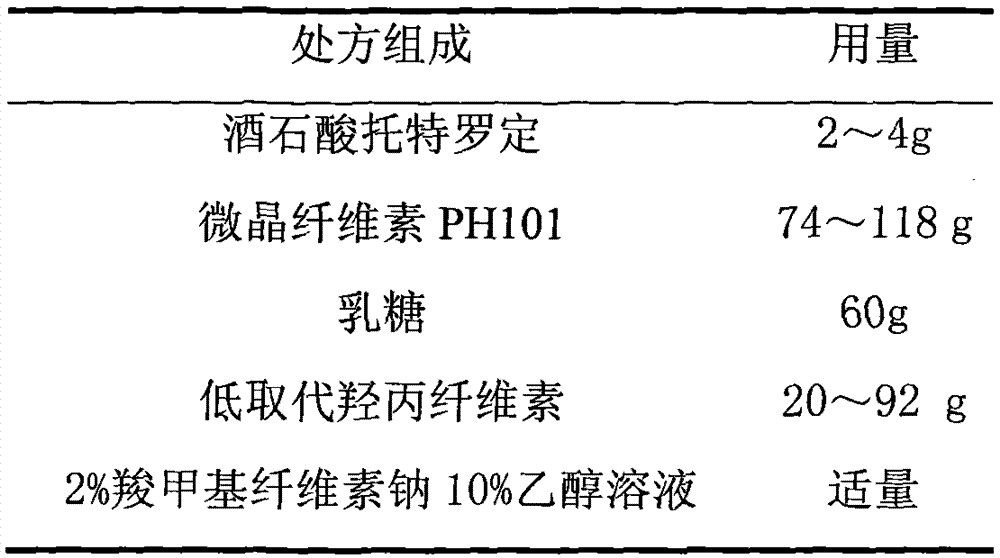
[0066] 1. Prescription
[0067] 1. Pill core prescription (1000 capsules)
[0068]
[0069] 2. Prescription of sustained-release film coating solution: same as in Example 1
[0070] 3. No. 0 stomach-soluble gelatin capsule shell 1000 capsules
[0071] Second, the preparation process:
[0072] 1. Ball core preparation process:
[0073] (1) the tolterodine tartrate of prescription quantity is mixed with lactose equal amount progressively;
[0074] (2) Weigh the microcrystalline cellulose PH101 of recipe quantity, low-substituted hydroxypropyl cellulose, the mixture of tolterodine tartrate and lactose mixed above, put in the wet granulator and mix evenly;
[0075] (3) 2% carboxymethylcellulose sodium 10% ethanol solution to make soft materials;
[0076] (4) Extrude on the extruder, the screen aperture is 1.0mm, and the extrusion speed is 20-30rp...
Embodiment 3
[0096] Example 3 Tolterodine tartrate sustained-release pellet capsule containing 20% low-substituted hydroxypropyl cellulose
[0097] 1. Prescription
[0098] 1. Pill core prescription (1000 capsules)
[0099]
[0100] 2. Prescription of sustained-release film coating solution: same as in Example 1
[0101] 3. No. 0 stomach-soluble gelatin capsule shell 1000 capsules
[0102] Second, the preparation process:
[0103] 1. Ball core preparation process: the same as in Example 2
[0104] 2. Preparation process of sustained-release film coating solution:
[0105] Same as Example 1
[0106] 3. Coating (sustained release film):
[0107] The ball core is placed in a fluidized bed for coating, and the weight gain of the coating film is controlled, and the weight gain of the coating is 25.1% and 28.8%.
[0108] 4, heat treatment: with embodiment 1
[0109] 5. Filling capsules:
[0110]The coated pellets are filled into capsules to obtain tolterodine tartrate sustained-rel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com