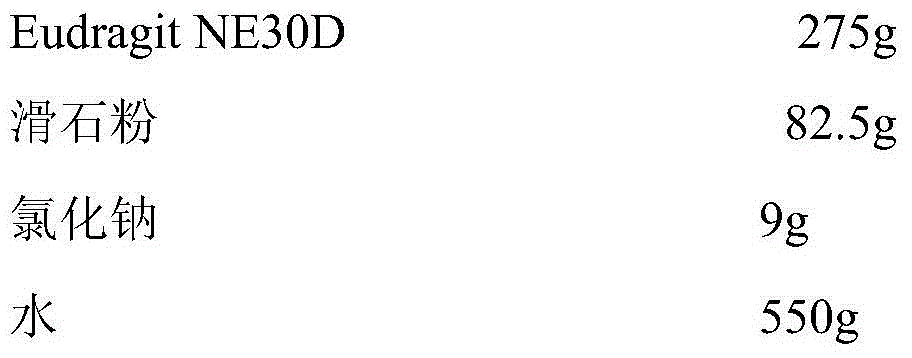Venlafaxine hydrochloride pellet capsule and preparation method thereof
A technology of venlafaxine hydrochloride and sustained-release pellets, applied in capsule preparations, venlafaxine hydrochloride sustained-release pellets capsules and their preparation fields, can solve the problems of venlafaxine hydrochloride being released too quickly and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A venlafaxine hydrochloride sustained-release pellet capsule, comprising a capsule shell and drug-containing pellets placed in the capsule shell, said drug-containing pellets comprising a drug-containing pellet core and a sustained-release capsule wrapped outside the drug-containing pellet core. layer, where:
[0035] Prescription with pill core:
[0036]
[0037] Sustained release layer prescription:
[0038]
[0039] The preparation method is as follows:
[0040] Mix water, cellulose, sucrose and venlafaxine hydrochloride evenly, and extrude the soft material into an extruder (screen diameter: 0.7mm, extrusion speed: 40rpm), and put the extruded material into a spheronizer ( The rotating speed is 300-2200rpm, and the spheronization time is 2 minutes). The spheronized extruded material is dried in a forced air drying oven at 40°C-60°C for 2-4 hours, sieved, and 16-35 mesh pellet cores are taken.
[0041] Coating solution preparation method: add talc and sodium...
Embodiment 2
[0044] A venlafaxine hydrochloride sustained-release pellet capsule, comprising a capsule shell and drug-containing pellets placed in the capsule shell, said drug-containing pellets comprising a drug-containing pellet core and a sustained-release capsule wrapped outside the drug-containing pellet core. layer, where:
[0045] Prescription with pill core:
[0046]
[0047] Sustained release layer prescription:
[0048]
[0049]
[0050] The preparation method is as follows:
[0051] Mix water, cellulose, lactose, sucrose and venlafaxine hydrochloride evenly. The soft material is extruded in an extruder (screen diameter: 0.7mm, extrusion speed: 40rpm), and the extruded material is spheronized in a spheronizer (rotating speed: 300-2200rpm, spheronization time: 2 minutes). Dry the material in a blast drying oven at 40°C-60°C for 2-4 hours, sieve, and take 16-35 mesh pellet cores.
[0052] Coating solution preparation method: Add talc and hydroxypropyl methylcellulose t...
Embodiment 3
[0055] A venlafaxine hydrochloride sustained-release pellet capsule, comprising a capsule shell and drug-containing pellets placed in the capsule shell, said drug-containing pellets comprising a drug-containing pellet core and a sustained-release capsule wrapped outside the drug-containing pellet core. layer, where:
[0056] Prescription with pill core:
[0057]
[0058] Sustained release layer prescription:
[0059]
[0060] The preparation method is as follows:
[0061] Mix water, cellulose, starch, sucrose and venlafaxine hydrochloride evenly. The soft material is extruded in an extruder (screen diameter: 0.7mm, extrusion speed: 40rpm), and the extruded material is spheronized in a spheronizer (rotating speed: 300-2200rpm, spheronization time: 2 minutes). Dry the material in a blast drying oven at 40°C-60°C for 2-4 hours, sieve, and take 16-35 mesh pellet cores.
[0062] Coating solution preparation method: add talc and polyethylene glycol to water and stir evenly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com