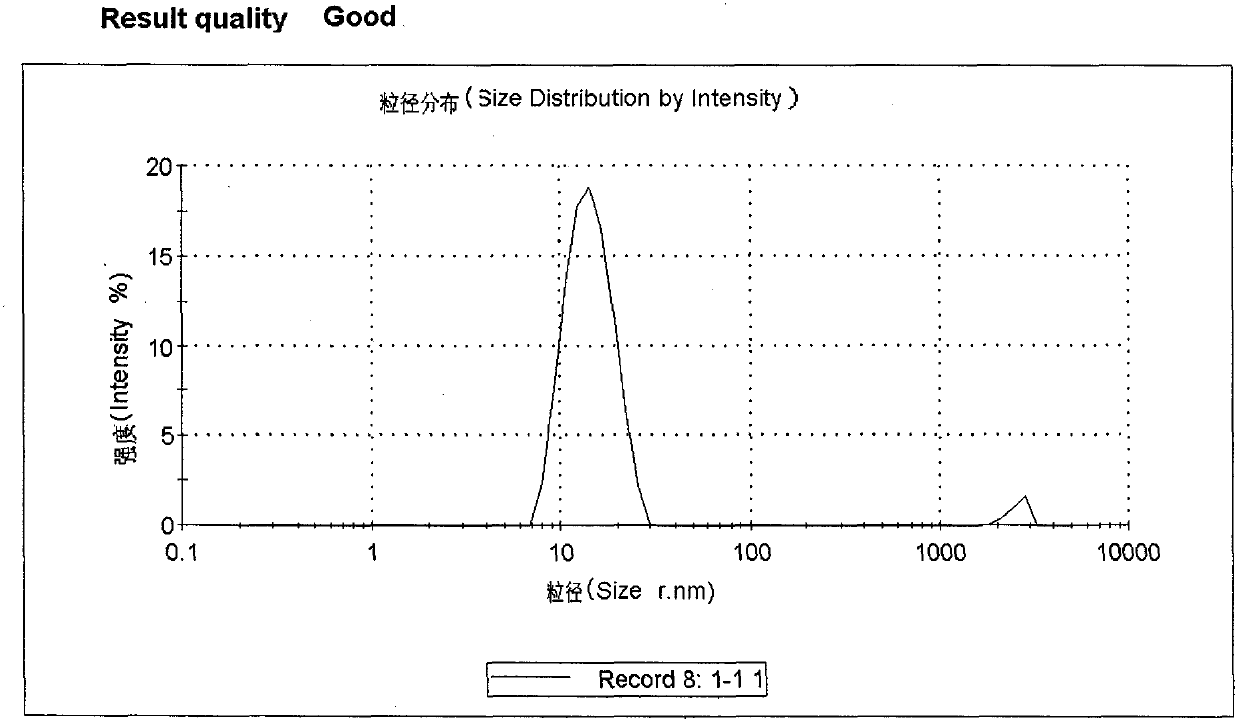
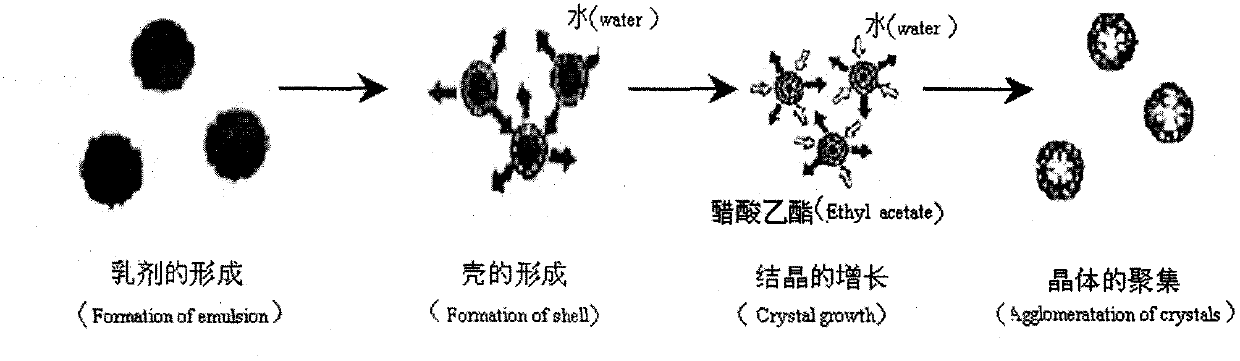
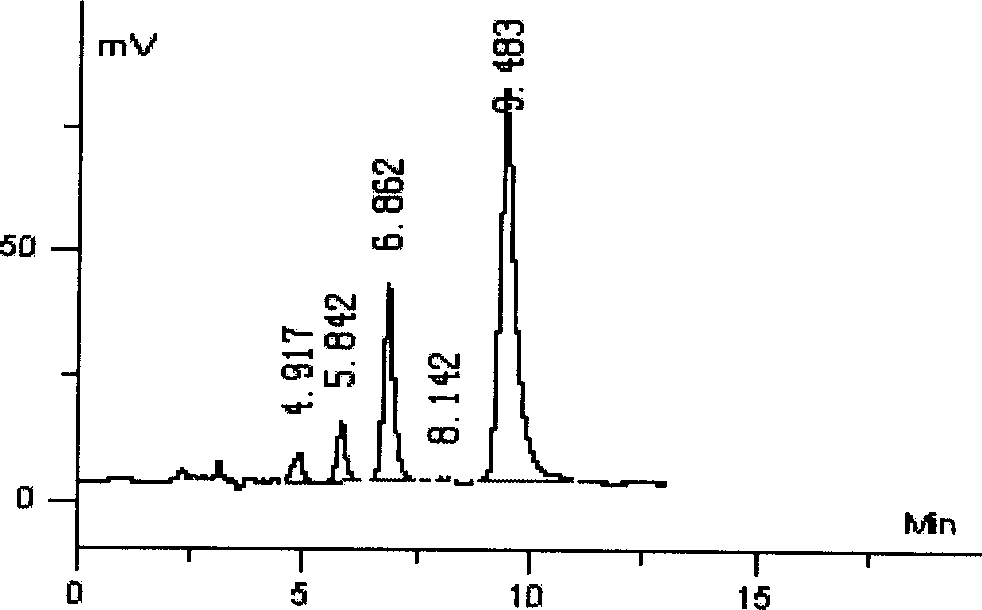
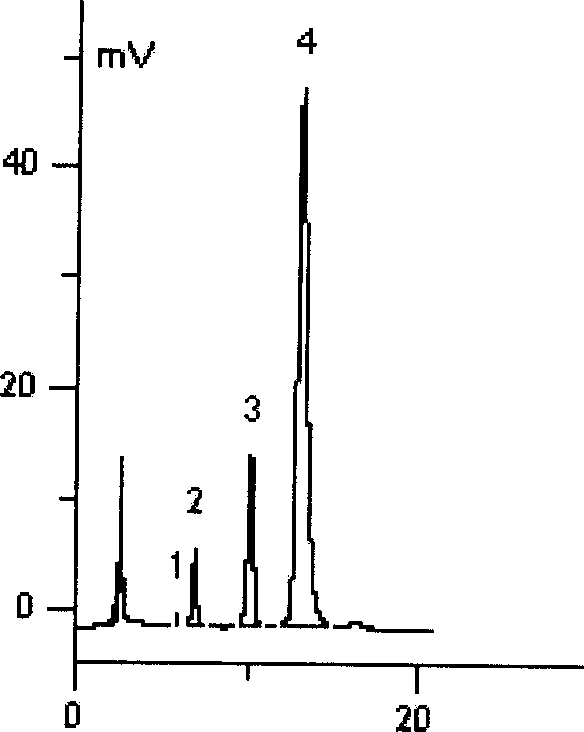
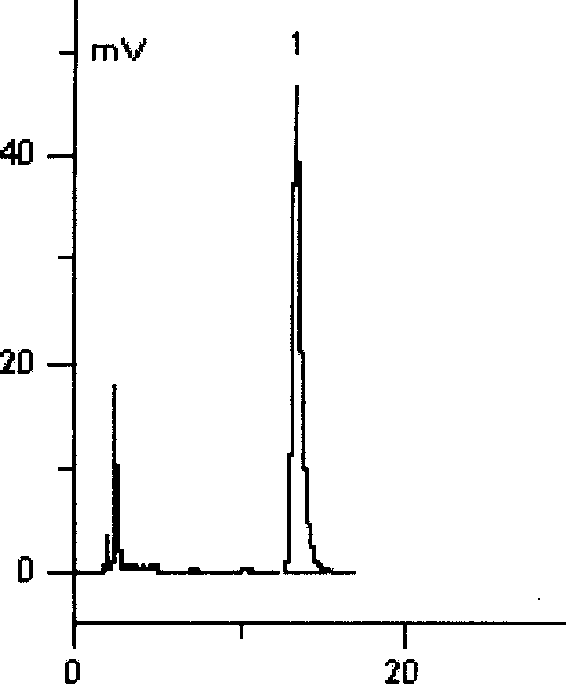
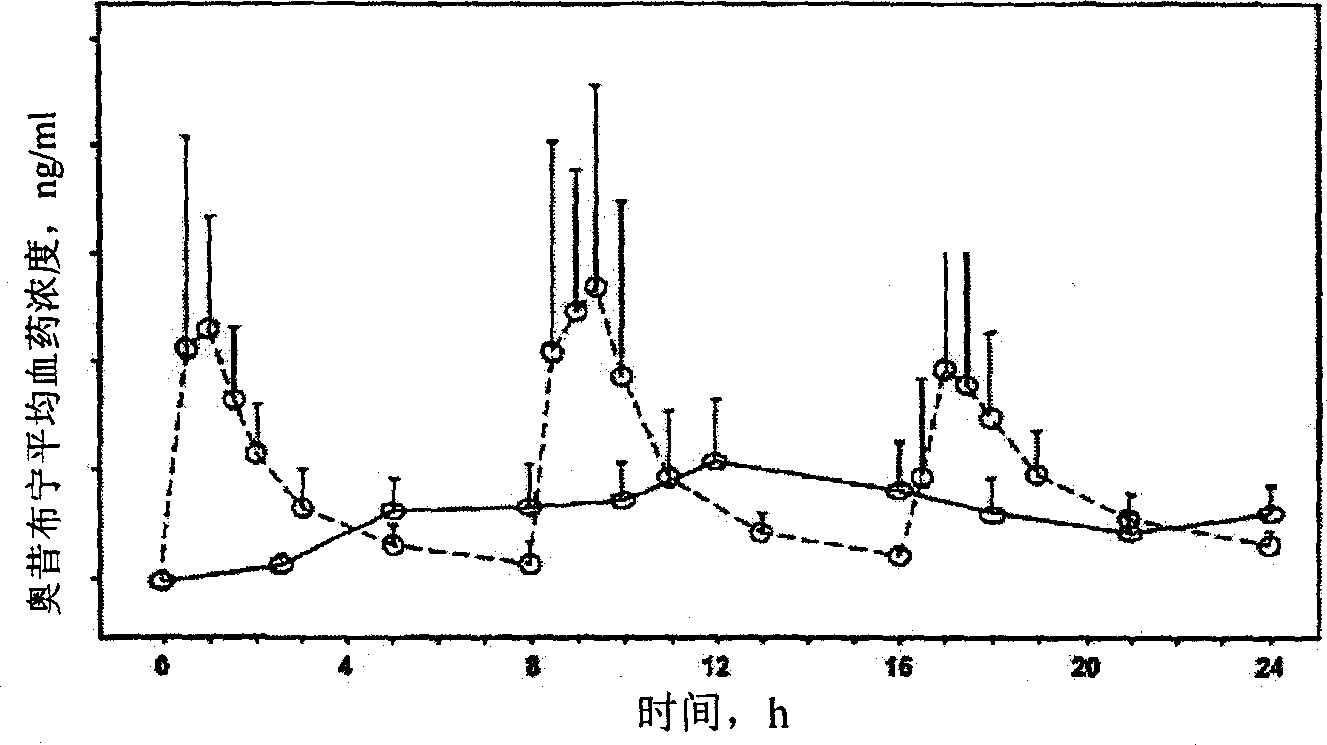
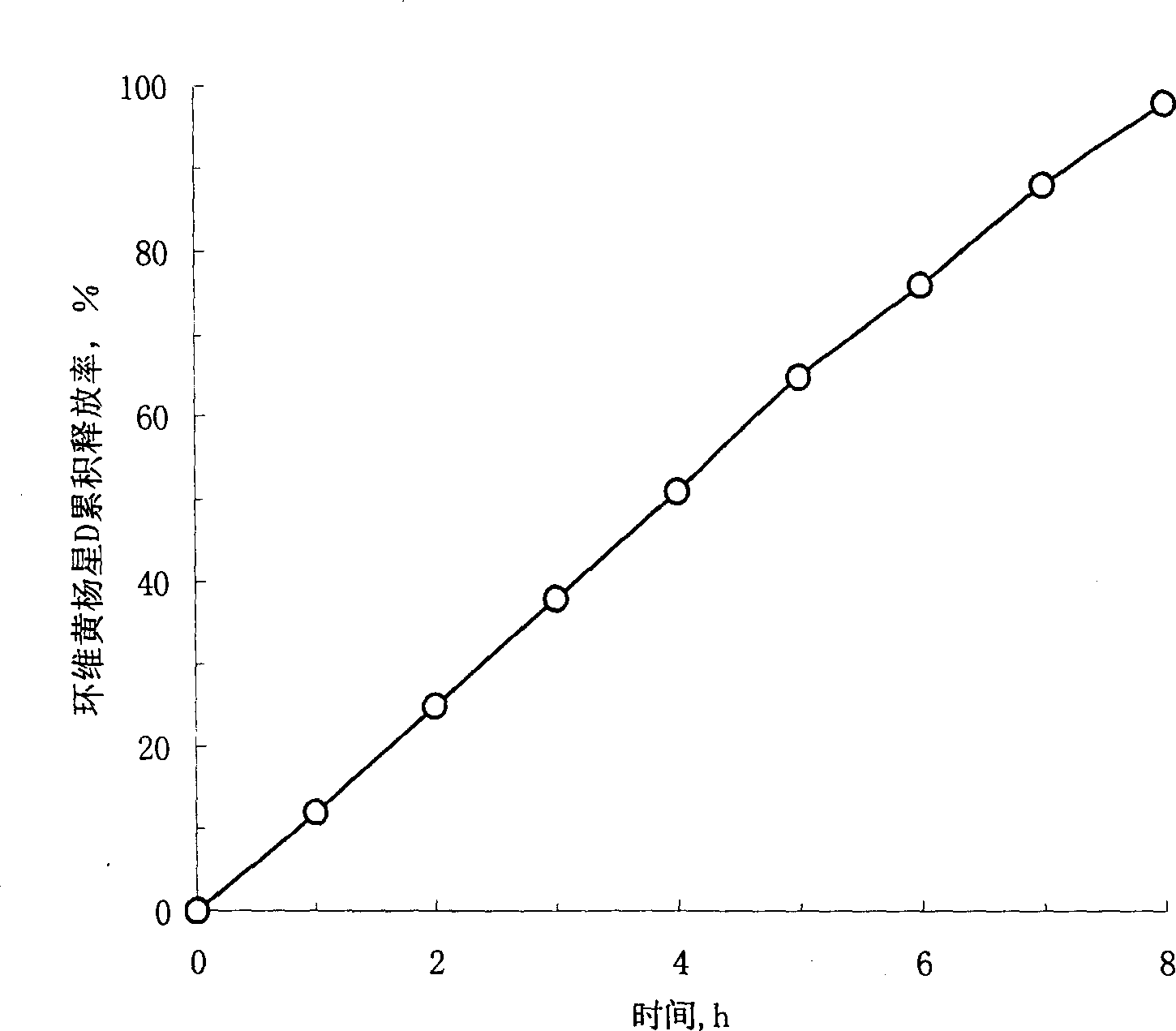
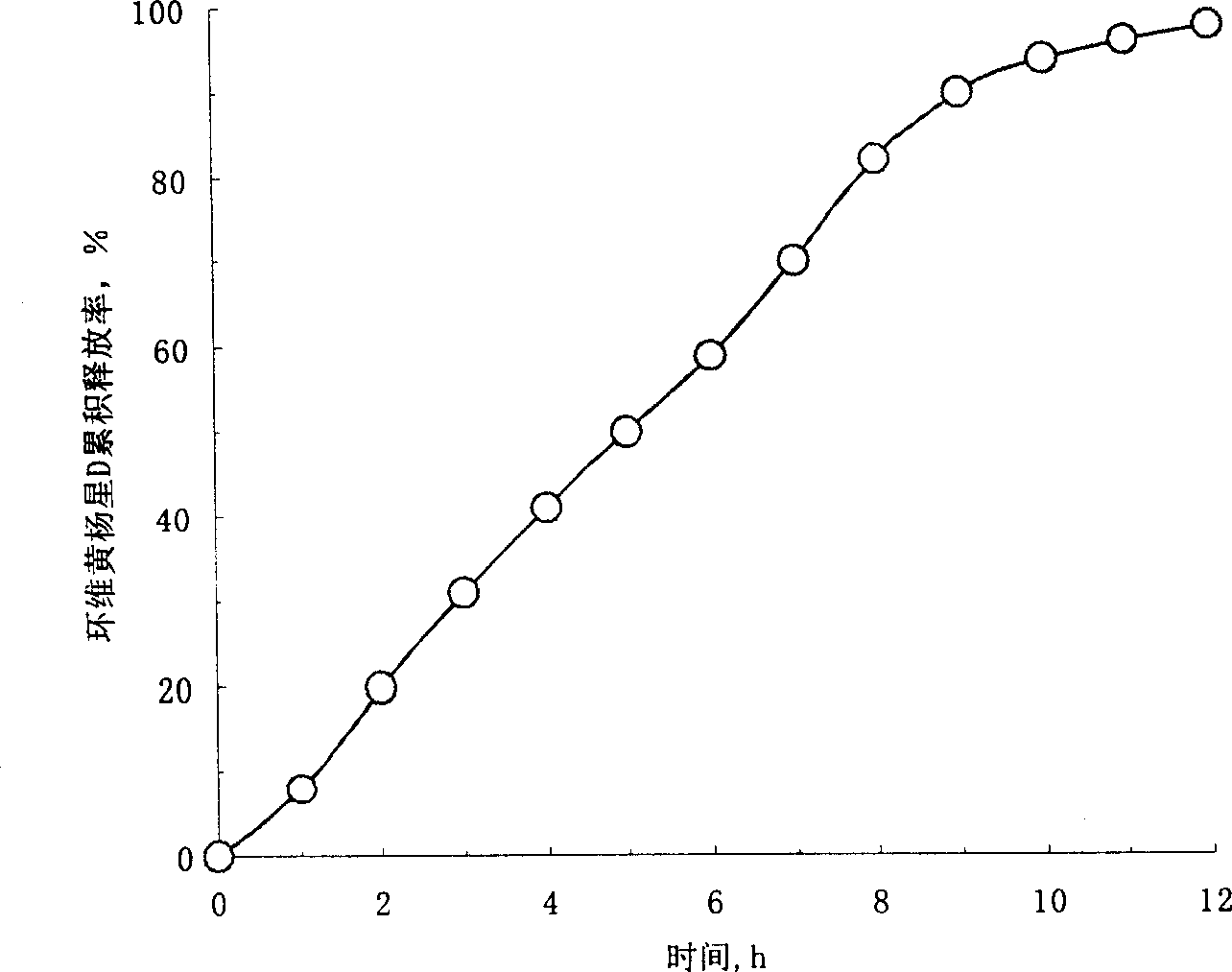
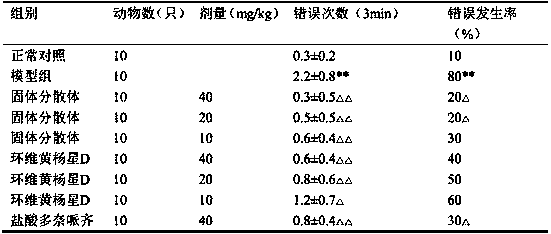
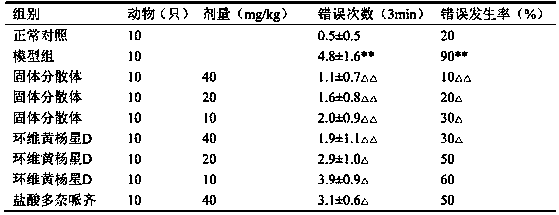
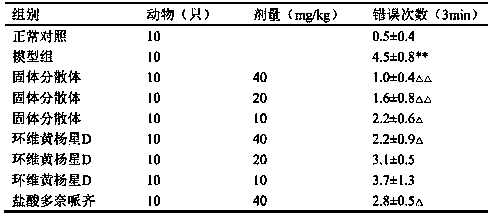
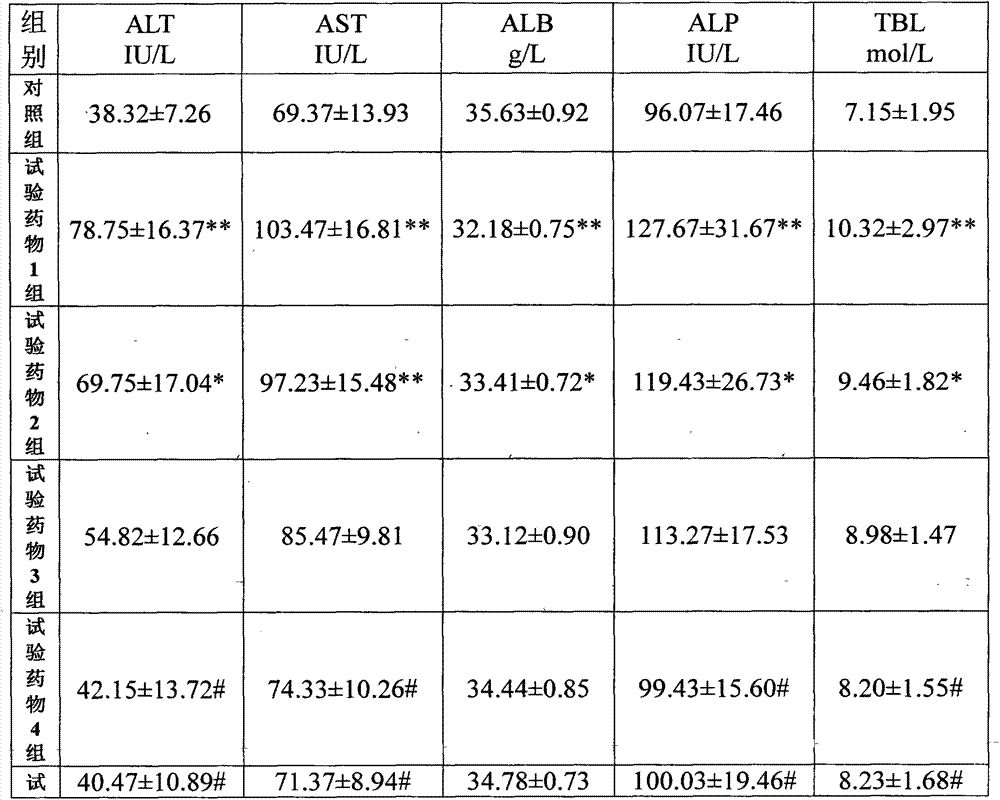
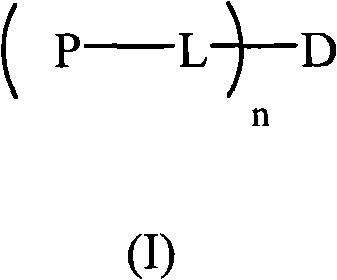Patents
Literature
52 results about "Cyclovirobuxine D" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Composition for treating skin pigmentation and related methods
A composition for treating skin pigmentation, a method of forming the composition, and a method of treating skin pigmentation are disclosed. The composition comprises at least one pharmaceutically acceptable additive. The composition further comprises at least one melanin production inhibitor, which may be a depigmentation agent. The melanin production inhibitor is present in an amount effective to inhibit melanin production in human skin cells of a subject that is administered the composition. The melanin production inhibitor is selected from the group consisting of cyclovirobuxine D, lipoic acid, nuciferine, peimisine, oleuropein, Rhodiola algida extract, Rhodiola kirilowii (Regel) Maxim extract, Glycyrrhizae Radix et Rhizoma extract, Cirsii japonici herba extract, Platycladi semen extract, Synotis erythropappa extract, Astragali complanati semen extract, Plantaginis semen extract, Lycopi herba extract, Euryales semen extract, Cuscutae semen extract, Amomi fructus extract, Tara seed extract, Platycladi cacumen extract, Eucommiae cortex extract, Dioscoreae rhizoma extract, Cirsii herba extract, and combinations thereof.
Owner:ACCESS BUSINESS GRP INT LLC
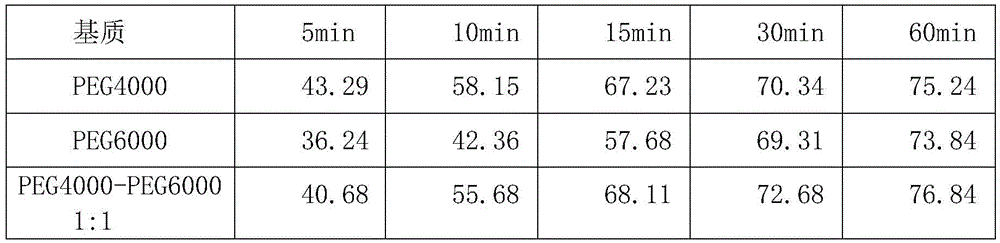
Solid self-microemulsion based on spherical crystallization technique and preparation method thereof
InactiveCN103315960AReduce liver and kidney toxicityAvoid influencePowder deliveryEmulsion deliveryNeogambogic acidCaprylic acid
The invention relates to the medical technology field, and particularly relates to a solid self-microemulsion based on a spherical crystallization technique and a preparation method thereof. The solid self-microemulsion is characterized in that: with the use of the spherical crystallization technique, the solid self-microemulsifying micoparticles are prepared from poorly water soluble drugs in a liquid phase by one step. The solid self-microemulsion with the poorly water soluble drugs comprises the components, by weight: 0.1 to 1.5 g of the poorly water soluble drugs, 4.0 g of a polyoxyethylene hydrogenated castor oil, 2.0 g of capric caprylic triglyceride, 2.0 g of tpropylene glycol, 1.0 ml of ethanol, 4.0 ml of dichloromethane, 0.5 to 1.1 g of ethylcellulose (or Eudragit RS100, RL100), 0.05 g of PEG4000, and 0.5 g of colloidal silicon dioxide. The poorly water soluble drugs include cyclosporine A, fenofibrate, glimepiride, cilnidipine, isradipine, simvastatin, baicalein, neogambogic acid, puerarin, cyclovirobuxine D, silymarin and the like.
Owner:胡容峰
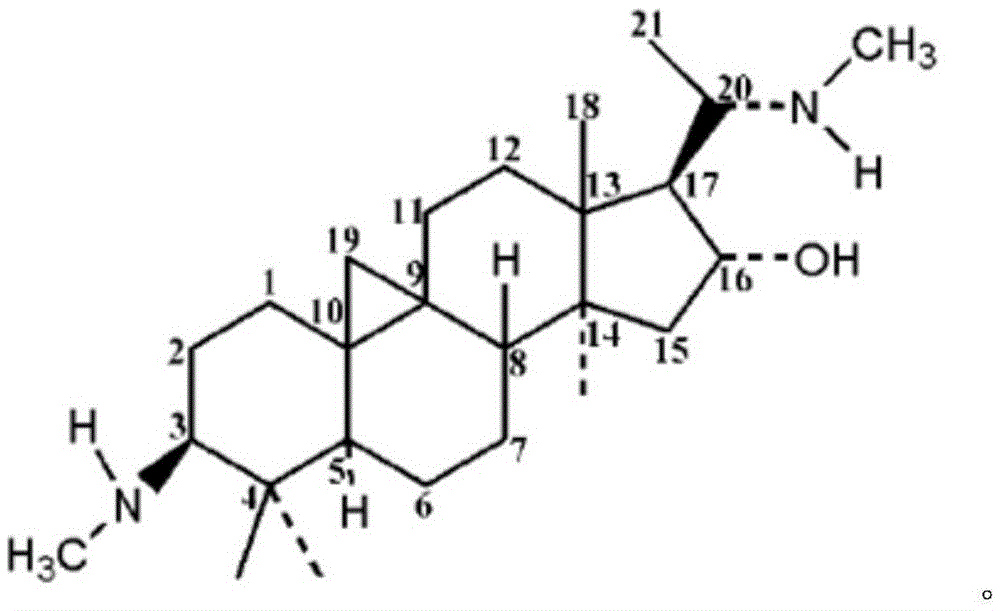
Buxine, buxine hydrochloride, and its preparing method and formulation
InactiveCN1814616AClear structureStable contentPowder deliveryOrganic active ingredientsMedicineQuality control
The invention discloses buxine. It is made up of cyclovirobuxine D, cyclovirobuxine D, and cyclovirobune C. And their weight content respectively is 60%-95%, 1.0%-30%, and 0.5%-20%. It also discloses buxine hydrochloride and its preparation method and their preparation. The formed product has clear composition and structure, effective quality control to ensure stable curative effect and easy to form various modern preparations. Its preparation technology is simple. And its cost is low.
Owner:杭太俊 +6
Osmosis pump controlled release preparation contg. Chinese medicine of cyclovirobuxine D, and preparing method thereof
InactiveCN1435178AImprove securityEasy to useOrganic active ingredientsPharmaceutical delivery mechanismControl releaseSemipermeable membrane
A controlled release medicine containing the Chinese-medicinal component cyclovirobuxine D is composed of the core tablet consisting of cyclovirobuxine D, penetrating active substance, acidic substance and high-molecular compound, and the coating film prepared from high-molecular compound. Its advantage is constant release speed.
Owner:BEIJING ZHONGHUI PHARM CO LTD
Cyclovirobuxine D salt and its preparation and use, and process for preparing said preparation
A cyclovirobuxine D salt, its injection for treating cardiovascular and cerebrovascular diseases, such as coronary heart disease and angine pectoris, its application and its preparing process are disclosed. Said injection is prepared from cyclovirobuxine D, hydrochloric acid or phosphoric acid, and glycose, sodium chloride, or mannitol.
Owner:西藏易明西雅医药科技股份有限公司
Medicinal composition for treating ischemic cerebrovascular disease, and preparation method thereof
InactiveCN102389434AConducive to therapeutic effectClear ingredientsHydroxy compound active ingredientsCardiovascular disorderDiseaseBULK ACTIVE INGREDIENT
The invention relates to a medicinal preparation containing organic active ingredients, in particular to a medicinal composition for treating ischemic cerebrovascular disease. The medicinal composition consists of active ingredients and medicinally acceptable auxiliary materials and is characterized in that the active ingredients consist of the following organic components in part by weight: 5 to10 parts of cyclovirobuxine D, 30 to 50 parts of ligustrazine, and 2 to 5 parts of borneol. In the medicinal composition, the cyclovirobuxine D is compatible with the ligustrazine and has an obvious synergic effect, and pharmacokinetics behaviors of the mechanical composition are changed by using borneol, so that bioavailability of the mechanical composition is improved obviously and the therapeutical effect of the medicine can be exerted conveniently.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Hydrophilic polymer-boxwood extract conjugate and its medicine composition
InactiveCN101306203AImprove stabilityGood water solubilityOrganic active ingredientsSolution deliverySolubilityPolyvinyl alcohol
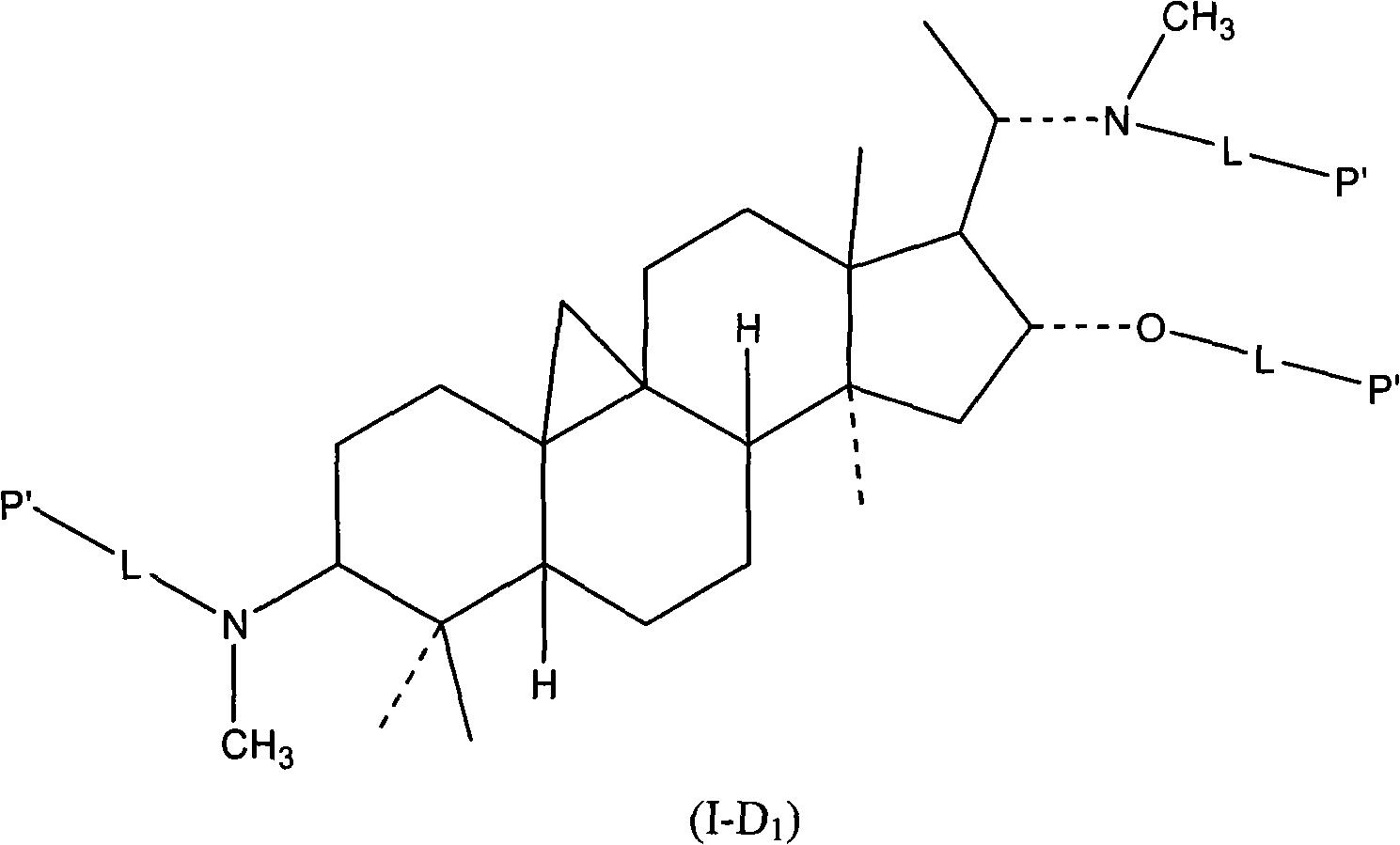
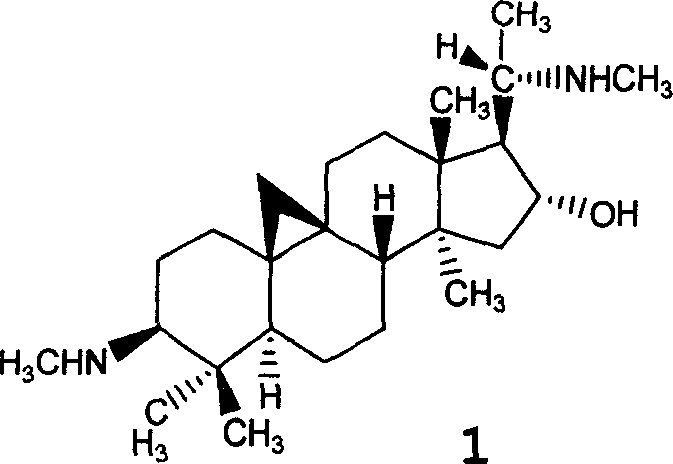
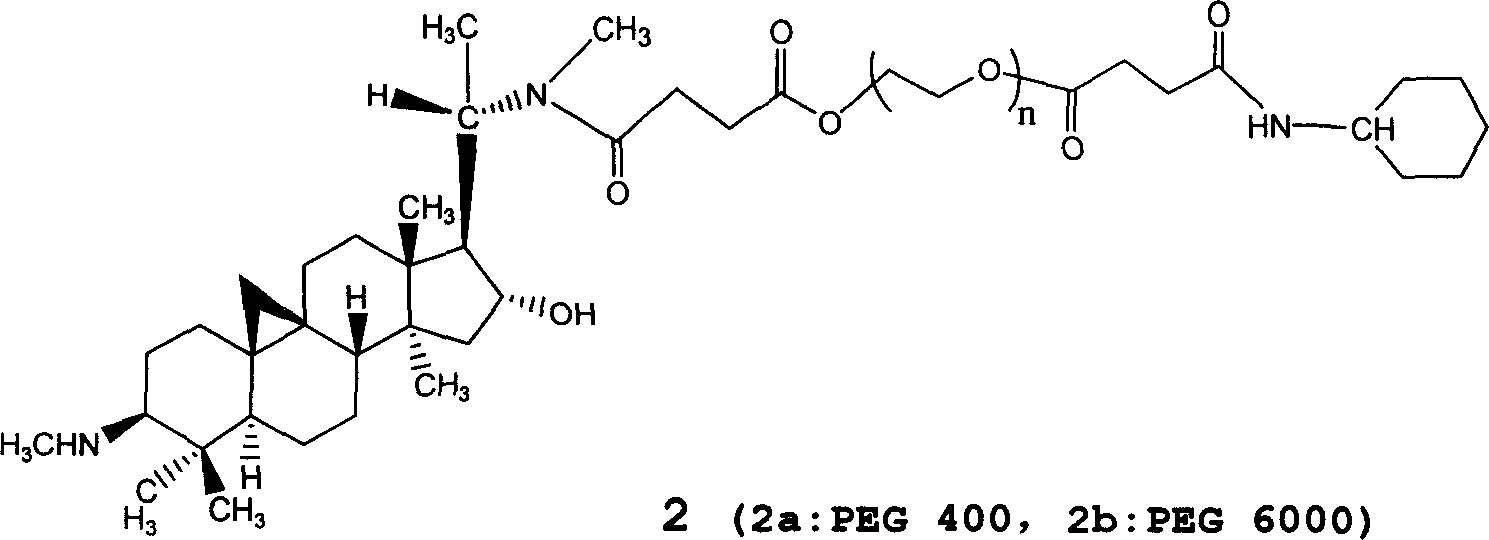
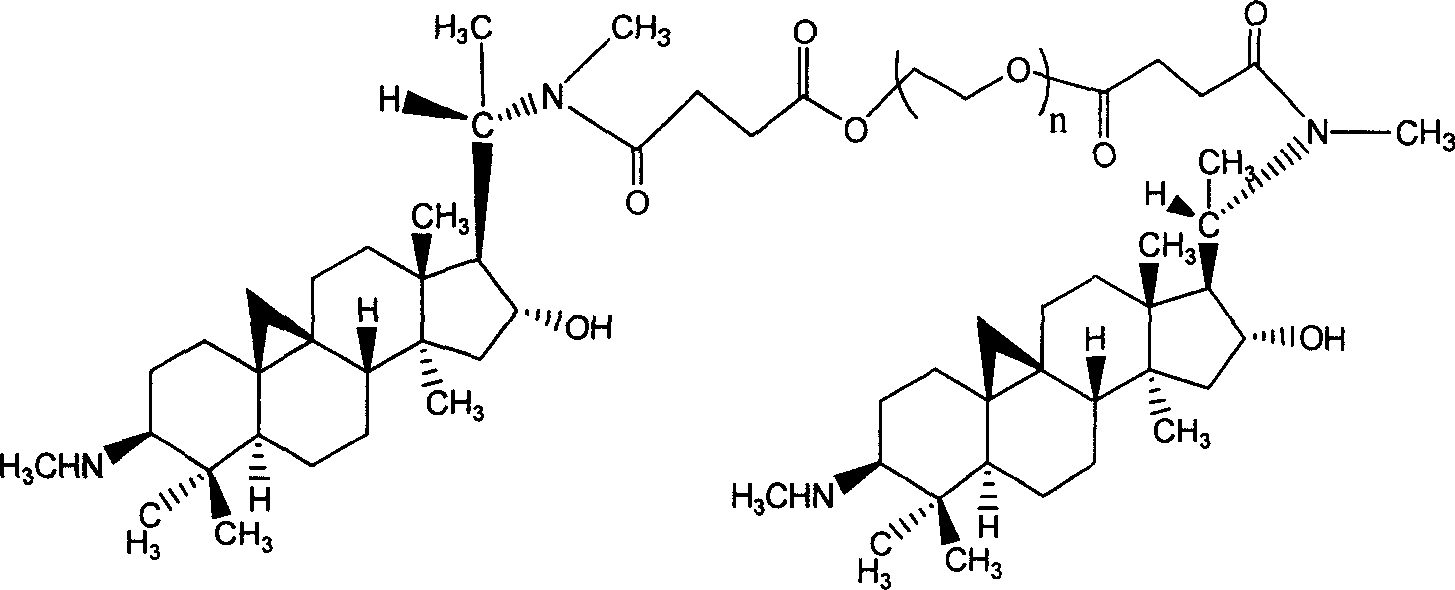
The invention relates to a hydrophilic polymer-boxwood alkaline extractive or a combination of a derivative thereof which is shown in a general formula (1), wherein, P is the hydrophilic polymer, which is selected from polyethylene glycol, polyglutamic acid, polyaspartic acid, polypropylene glycol, polyvinyl alcohol, polypropylene morpholine and a copolymer thereof; n is an integer, which does not exceed the total quantity of hydroxyl group and amino group on the D; L is a linking group, which is selected from groups formed by ester group, carbonate group, amide group, amide ester group, ether group, urethane group and acetal; D is the boxwood alkaline extractive or the derivative thereof, which is selected from groups formed by cyclovirobuxine D, cycloprotobuxine A, cycloprotobuxine C, cyclovirobuxine C and derivatives thereof. The combination improves the water solubility of boxwoods, and prolongs the cycle half-life thereof in the organism.
Owner:JENKEM TECH CO LTD TIANJIN
Method for increasing solubility of parabuxinidine-D injection
InactiveCN1456165AGood water solubilityHigh yieldOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityFreeze-drying
A method for improving the solubility of cyclovirobuxine D injection in the form of liquid, freeze dried injection, sodium chloride injection, or glucose injection is prepared through mixing cyclovirobuxine D with acidic substance, adding excipient to obtain powder, holding pH value to 1-6.5, and stirring. Its advantages are high solubility in acidic solution (up to 100%), low cost and high curative effect.
Owner:郭曙平
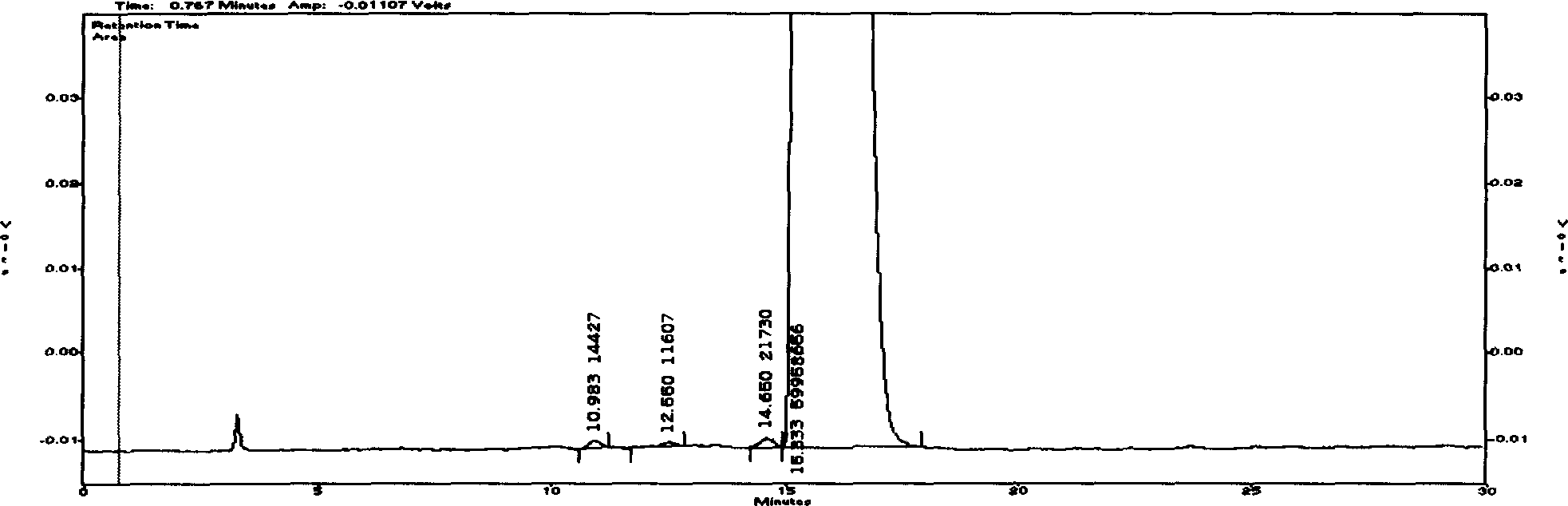
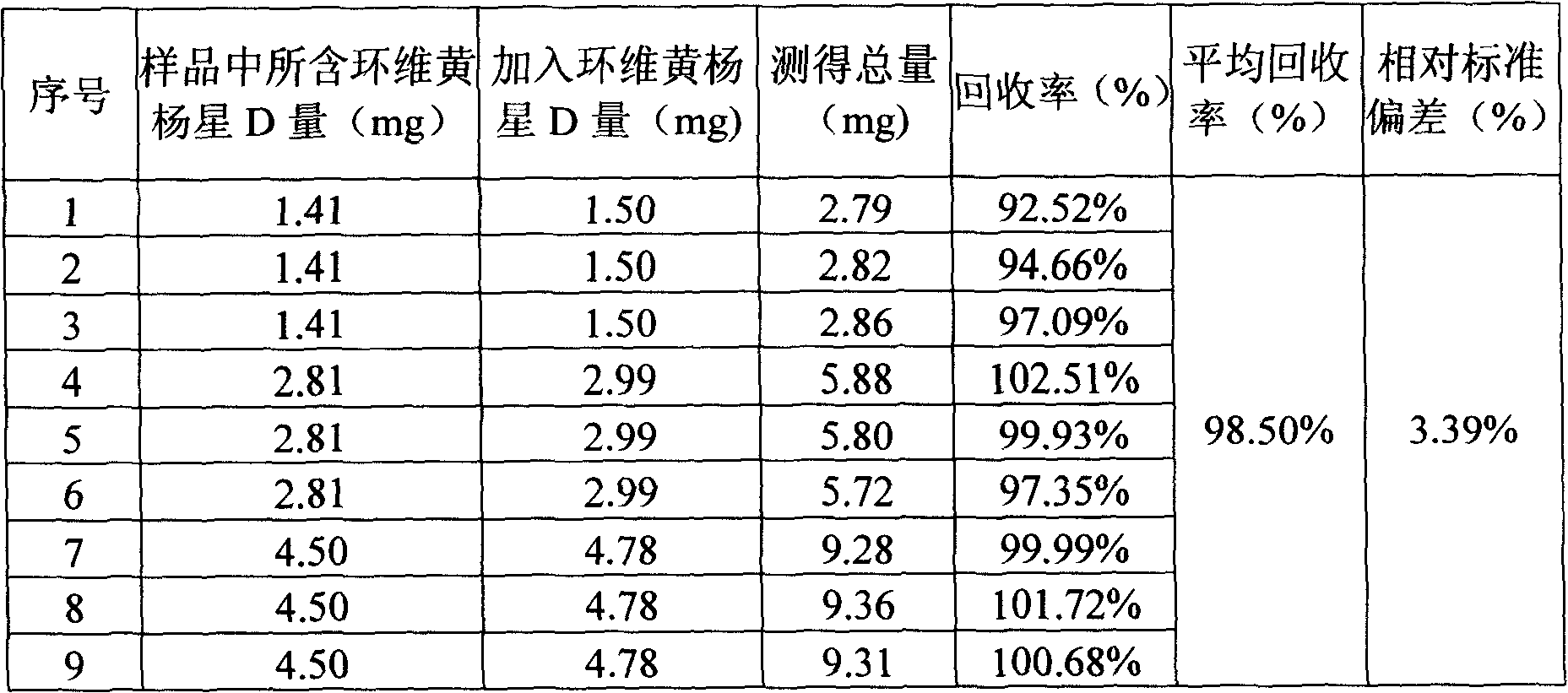
Cyclovirobuxine D raw medicine and method for determining its content in preparation by chromatography
The invention discloses a Cyclovirobuxine-D raw material drug and high efficiency liquid chromatography mensuration for Cyclovirobuxine-D content in different kinds of preparation, and high efficiency liquid chromatography for impurity testing in Cyclovirobuxine-D. The fixed phase in chromatographic condition uses octadecyl silane linkage silica gel or octyl silane linkage silica gel as filling material; The mobile phase adopt formate (ammonium formate) damping fluid-methanol system, acetic acid (ammonium acetate) damping fluid-methanol system, formate (ammonium formate) damping fluid-acetonitrile system or acetic acid (ammonium acetate) damping fluid-acetonitrile system; Evaporation light scattering detector (ELSD) detects drift tube temperature; drift tube temperature: 20-150 degree centigrade;and gas flow rate: 0.1L / min- 6.0L / min, split-flow or not split stream sampling. The method is easy to operate, timesaving, and has strong expert attribute. It could effectively divide some kind of alkaloids that has with Cyclovirobuxine-D in Cyclovirobuxine-D raw material drug. Thus, it is able to accurately measure their content. The method could also detecting impurity in Cyclovirobuxine-D. The invention could also measure content degree of homogeneity of Cyclovirobuxine-D in boxwood-nin piece, boxwood-nin drop pill, and boxwood-nin powder pin. And it commendably satisfies the request of pharmacopoeia content and content degree of homogeneity testing.
Owner:TIANJIN TASLY PHARMA CO LTD
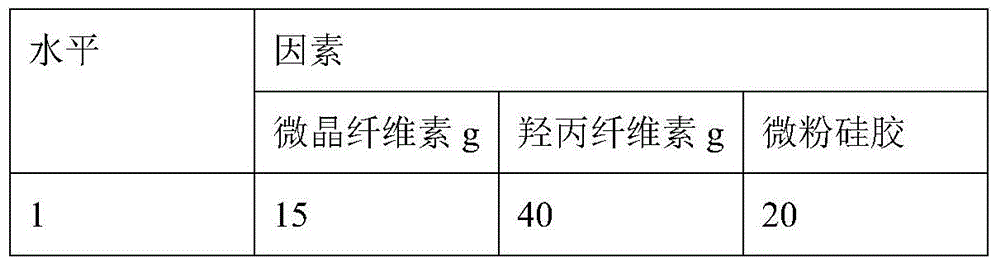
Huangyangning dispersible tablets and preparation method thereof
The invention discloses Huangyangning dispersible tablets and a preparation method thereof. Raw materials are subjected to nanocrystallization with a supercritical fluid method, then Cyclovirobuxine D, soybean lecithin, PEG4000, microcrystalline cellulose, hydroxypropyl cellulose, aerosil and aspartame are granulated with an equivalent incremental method by use of 60% ethyl alcohol, and drying and tableting are performed. Compared with Huangyangning on the market, the dispersible tablets have characteristics of high solubility and dissolution rate, quick absorption, high bioavailability and the like; the quality of the dispersible tablets can be better improved and development of drugs is facilitated.
Owner:INCREASE PHARMA YINGKOU CO LTD
Salt of organic acid of cyclorrirobuxin-D, pharmaceutics and application as well as its preparation method
InactiveCN1519246AEasy to useQuick effectOrganic active ingredientsAntipyreticDiseaseButanedioic acid
An organic acid salt of cyclovirobuxine D in the form of injection for treating cardiovascular and cerebrovascular diseases including coronary heart disease, arrhythmia, etc. is prepared from the cyclovirobuxine D, citric acid or tartaric acid or lactic acid or butanedioic acid or methanesulfonic acid, and glucose or sodium chloride or mannitrol.
Owner:曹明成
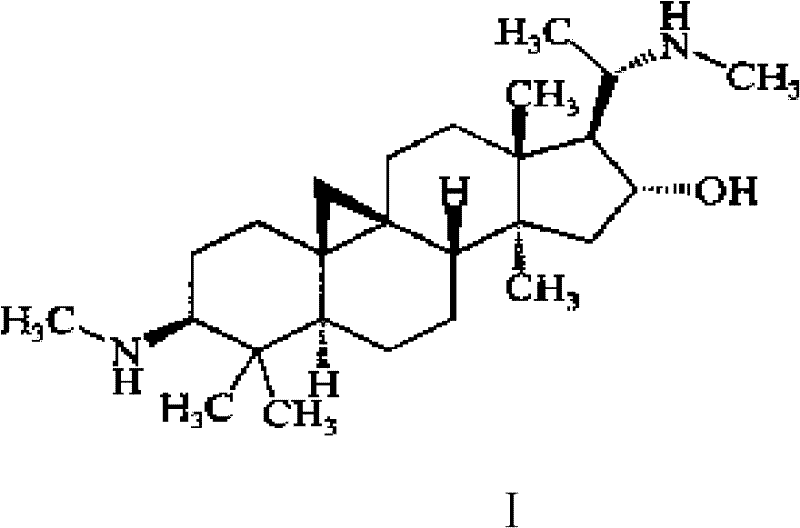
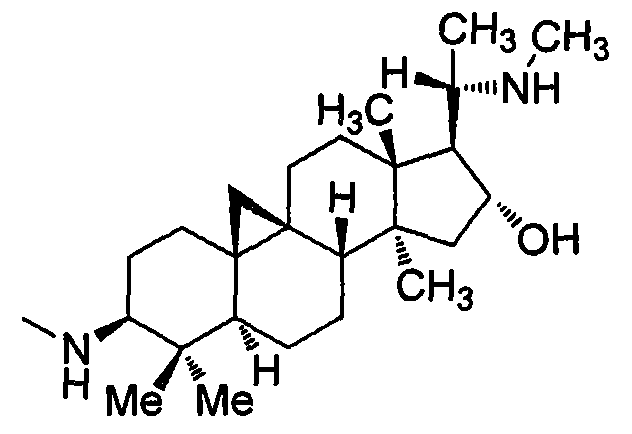
Cyclovirobuxine D derivative, and preparation and use thereof
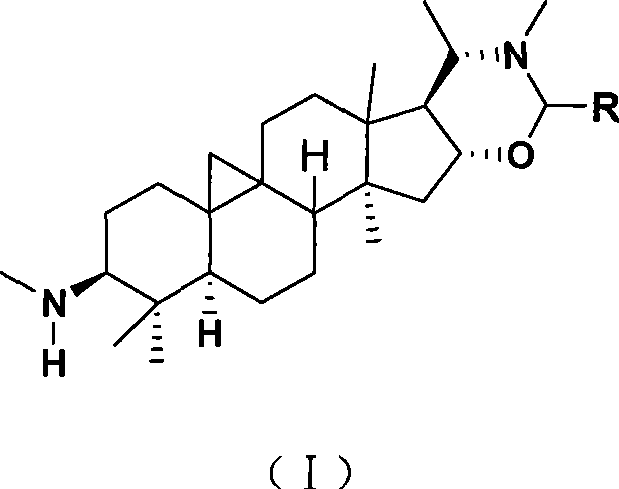
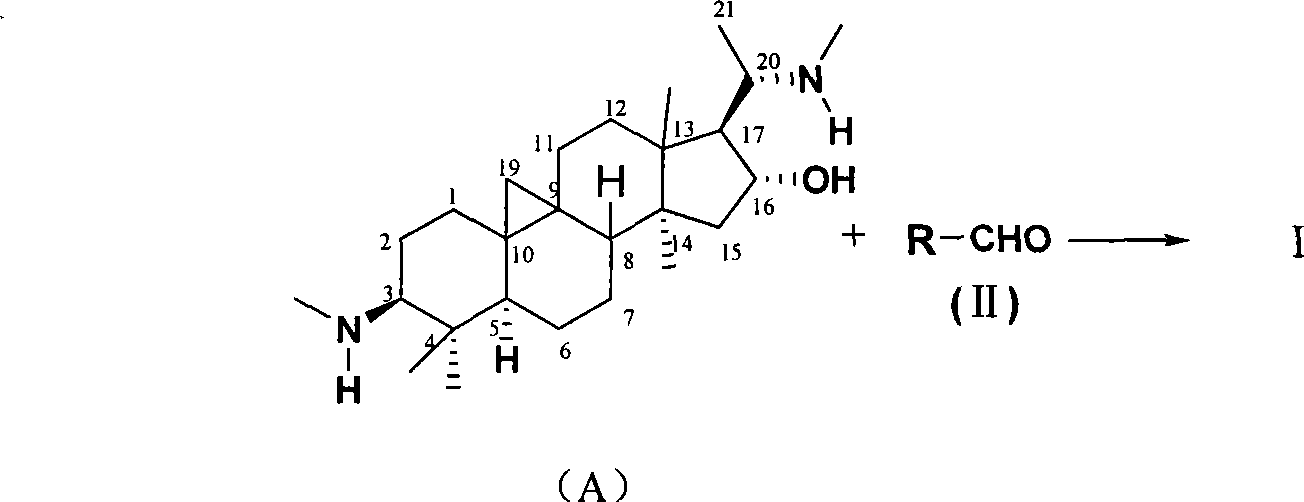
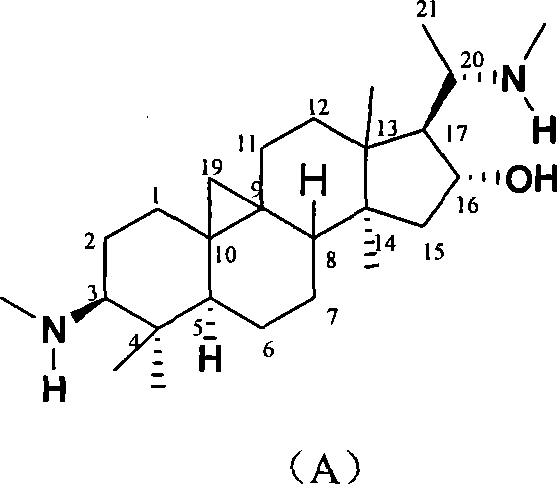
The invention discloses a cyclovirobuxine D derivative and a preparation method and the application thereof. The structure of the derivative is shown as formula (I): R in the formula is selected from H, C1-6alkyl, phenyl, p-nitrophenyl, para halogen phenyl, para-C1-6alkyl substituted phenyl, para-C1-6alkoxy, para-trifluoromethyl-phenyl, para-dimethylamino-benzene base, inter-nitrophenyl, 3, 4-dimethyl-phenyl, 3, 4, 5- trimethyl-phenyl, 3, 4-dimethoxy-phenyl, 3, 4, 5-trimethoxybenzaldehyde base, 3-thiazolyl, 2-furyl or 1-naphthyl. The derivative can be prepared by taking cyclovirobuxine D and aldehydes (II) as raw materials. The derivative has the same effect as the cyclovirobuxine D, can be combined with pharmaceutically-acceptable auxiliary components into injection agent which can be used for treating cardiovascular and cerebrovascular disease and various oral pharmaceutical preparations.
Owner:SICHUAN CHUANDA WEST CHINA PHARMA +1
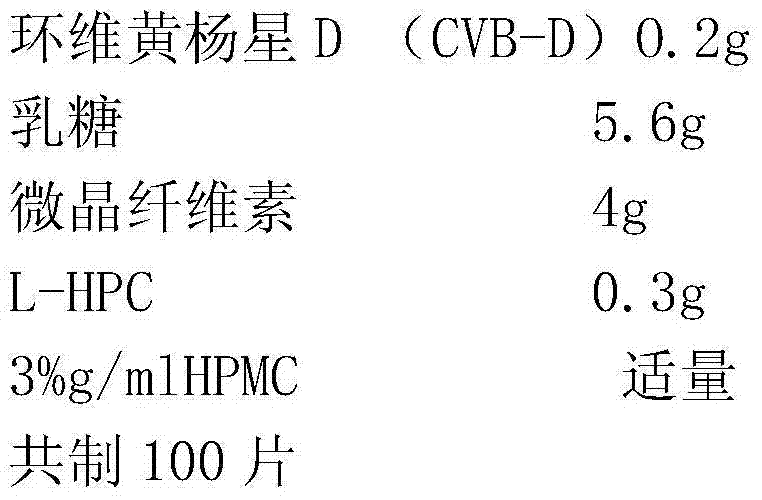
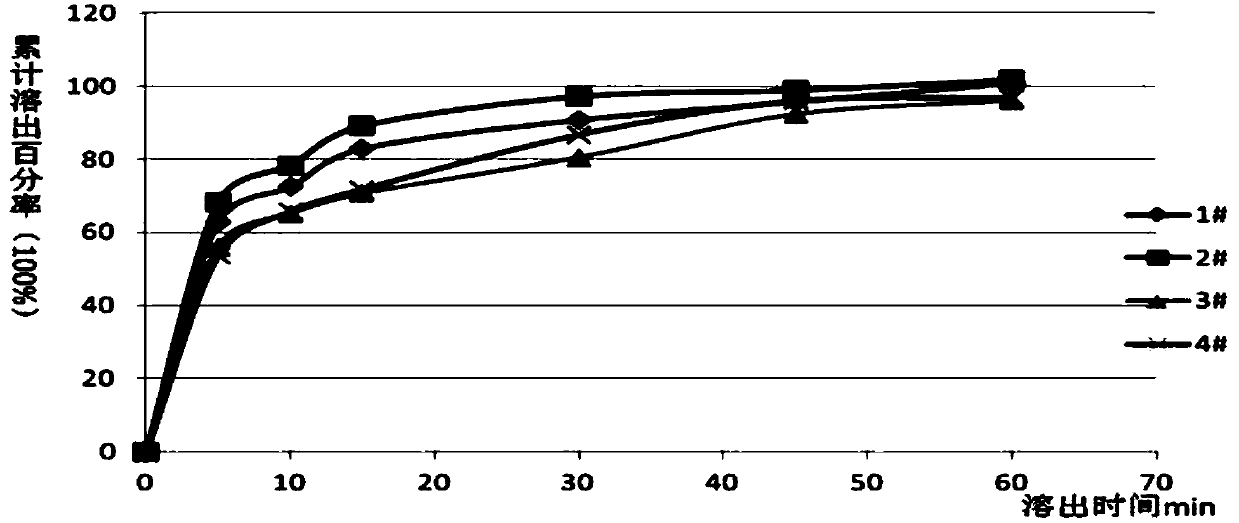
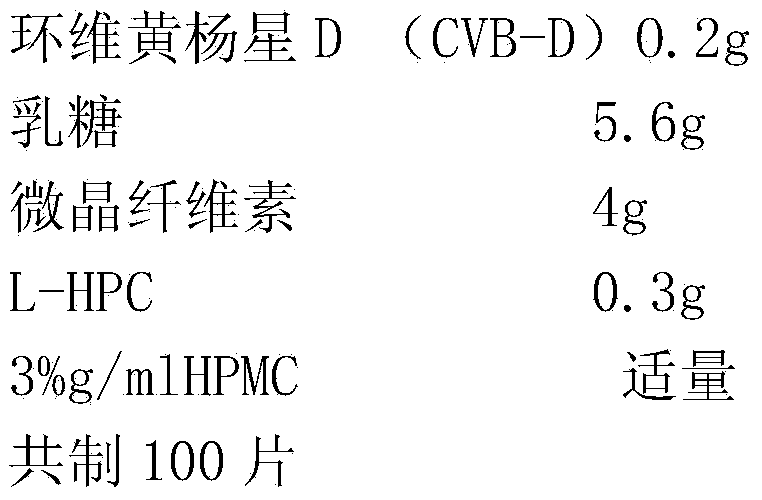
Cyclovirobuxine D sublingual tablet as well as preparation method and application thereof
InactiveCN103432090BReduce typesHigh drug contentOrganic active ingredientsNervous disorderMedicineLactose
The invention provides a cyclovirobuxine D sublingual tablet which is a preparation prepared from the following raw materials and auxiliary materials in parts by weight: 0.1-0.2 part of cyclovirobuxine D, 4-7 parts of lactose or pregelatinized starch, 3-5 parts of microcrystalline cellulose and 0.3-0.7 part of low-substituted hydroxypropyl cellulose. The invention further provides a preparation method and an application of the sublingual tablet. The sublingual tablet provided by the invention has small categories of auxiliary materials, high drug concentration, high disintegrating speed and excellent permeable membrane absorption, and can be effectively used for sublingual administration; in addition, after the raw materials and the auxiliary materials are mixed, a special dispersion technology is not needed for processing so that a forming process is simple and convenient, the production cost is low, and the industrial production is beneficial.
Owner:CHENGDU MEDICAL COLLEGE
Cyclovirobuxine D sublingual tablet as well as preparation method and application thereof
InactiveCN103432090AReduce typesHigh drug contentOrganic active ingredientsNervous disorderMedicineLactose
The invention provides a cyclovirobuxine D sublingual tablet which is a preparation prepared from the following raw materials and auxiliary materials in parts by weight: 0.1-0.2 part of cyclovirobuxine D, 4-7 parts of lactose or pregelatinized starch, 3-5 parts of microcrystalline cellulose and 0.3-0.7 part of low-substituted hydroxypropyl cellulose. The invention further provides a preparation method and an application of the sublingual tablet. The sublingual tablet provided by the invention has small categories of auxiliary materials, high drug concentration, high disintegrating speed and excellent permeable membrane absorption, and can be effectively used for sublingual administration; in addition, after the raw materials and the auxiliary materials are mixed, a special dispersion technology is not needed for processing so that a forming process is simple and convenient, the production cost is low, and the industrial production is beneficial.
Owner:CHENGDU MEDICAL COLLEGE
Method for determining content of cyclovirobuxine D
InactiveCN1888892AEasy to separateQuality assuranceComponent separationTesting medicinal preparationsAcetonitrilePhenylisocyanate
A High Performance Liquid Chromatography (HPLC) test method for testing the content of cyclovirobuxine-D in material and all kinds of dosage form (Huangyangningdiwan and so on) by pre-column deri-vatization, and the derivatizing reagent is isocyanic phenyl ester. Test each 0.2mg cyclovirobuxine-D with 1 mul / ml isocyanic phenyl ester solution 0.05- 0.6ml with the acetonitrile-water system as mobile phase with ultraviolet inspector. It can exactly test the content of cyclovirobuxine-D in material and all kinds of dosage form with easy operation, high precision, measurable dosage of derivatizing reagent and little side-effect.
Owner:GUANGZHOU CHEN LI JI PHARMA FACTORY
A kind of preparation method of Cyclovitamin D hydrochloride
The invention provides a preparation method for cyclovirobuxinum D hydrochloride. The preparation method comprises the following steps: subjecting a crude product of cyclovirobuxinum D to recrystallization by using one or more selected from a group consisting of methanol, ethanol and chloroform; and dissolving a recrystallization product in an organic solvent, adding 1M dilute hydrochloric acid drop by drop, carrying out a reaction at room temperature for salt formation, then subjecting a precipitated salt to filtering and drying, then subjecting the dried salt and a mixed solvent of methanol and ethanol to heating reflux, and carrying out slow and natural cooling for crystallization after a solution is clear so as to obtain cyclovirobuxinum D hydrochloride. The preparation method provided by the invention is low in cost, simple to operate, easy to realize large-scale production, and high in yield, wherein the yield of the salt formation reaction is 90% or above; and the prepared cyclovirobuxinum D hydrochloride has HPLC purity of more than 99%.
Owner:JIANGSU JINGLIXIN PHARMA TECH CO LTD
Method for improving purity of cyclovirobuxine D
The invention discloses a method for improving the purity of cyclovirobuxine D. The method comprises the following steps: (1), dividing a boxwood alkaloid raw material into secondary amine alkaloid and tertiary amine alkaloid by using a pH gradient extraction method or using methyl iodide and a lithium triethylborohydride reagent; (2), dissolving the secondary amine alkaloid in an organic solvent, adding a carbenoid reagent for a reaction, then adding palladium-carbon, introducing hydrogen for a reaction, or adding a nitroso acyl chloride reagent to the secondary amine alkaloid for a reaction so as to obtain high-purity cyclovirobuxine D; and (3), adding a cyanogen bromide reagent to the tertiary amine alkaloid, performing a Von Braun reaction so as to remove methyl groups in the tertiary amine alkaloid, or performing an iodine oxidation reaction on the tertiary amine alkaloid so as to remove the methyl groups in the tertiary amine alkaloid, and then performing column chromatography treatment so as to obtain the high-purity cyclovirobuxine D and cyclovirobuxine C. Through the method for improving the purity of the cyclovirobuxine D, not only can resources be saved, but also the purity of the cyclovirobuxine D can be improved.
Owner:荆门市锦秀天成医药技术服务有限公司
New cyclovirobuxine D purifying method
InactiveCN102993260ASimple processEasy to separateIon-exchange process apparatusOther chemical processesStationary phaseSilica gel
The invention relates to a new cyclovirobuxine D purifying method. A meso-porous silica gel surface molecular imprinting stationary phase is adopted as a filler, and a meso-porous silica gel is prepared through one-step copolycondensation of tetraethoxysilane, a template, an acid, salt, a cosolvent and 1,3,5-trimethylbenzene through a sol-gel method and has large apertures of 10-20nm, and the meso-porous silica gel surface molecular imprinting stationary phase treating cyclovirobuxine D as a template molecule is synthesized through mainly treating the meso-porous silica gel as a carrier and adopting a grafting copolymerization method. The meso-porous silica gel surface molecular imprinting stationary phase is filled into a chromatographic column to purify a bulk drug of the cyclovirobuxine D in order to obtain high-purity cyclovirobuxine D.
Owner:CHINA PHARM UNIV
First-class new medicine cyclovirobuxine D series for treating cardiovascular and cerebrovascular disease and method for preparing the same
InactiveCN1827116AGood blood compatibilityImprove physiological activityOrganic active ingredientsCardiovascular disorderDiseaseSolubility
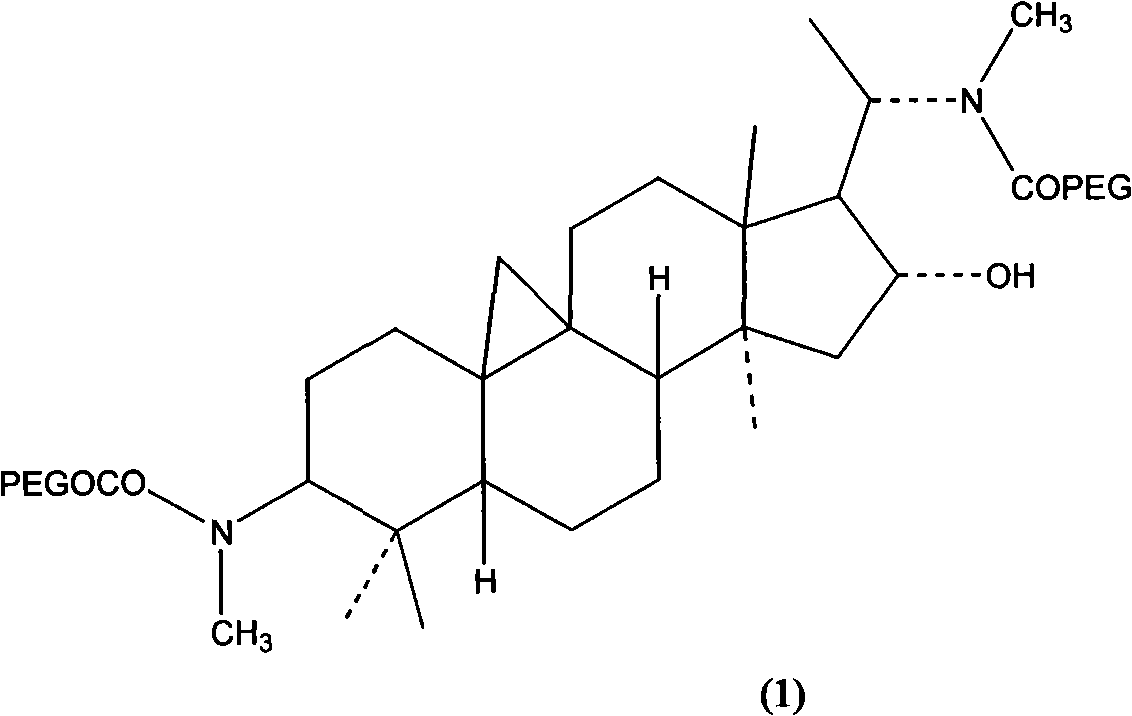
By organic synthesis and high molecular polymerization and other measures in this invention, graft hydrophilic polietilenglicol on the sideline of cyclovirobuxine D, and obtain a series of modified cyclovirobuxine D new drugs for cerebrovascular disease therapy. Characterize product structure with IR, 1H-NMR, GPC, MS, and so on; at the same time do its water-solubility, hemolysis test and hypoxia-resistant test on rat. The results above show: activated polietilenglicol has been successfully grafted on cyclovirobuxine D, and this turns water-insoluble cyclovirobuxine D into water-soluble polyaethylenglycolization cyclovirobuxine D; also it has good blood compatibility and physiologic activity. So the new drugs of polyaethylenglycolization cyclovirobuxine D system can be made into ampules, oral liquid, capsule, troche, and any other form feasible to clinic. The average molecular weight of the polietilenglicol is 200-60000.
Owner:SICHUAN CHUANDA WEST CHINA PHARMA +1
Pharmaceutical composition for treating atherosclerosis and application thereof
InactiveCN105998045AEffective treatmentSuitable for long-term useOrganic active ingredientsCardiovascular disorderAtheromaToxin
The invention belongs to a pharmaceutical composition for treating atherosclerosis and its application; the pharmaceutical composition is prepared from the following raw materials: cyclovir buxicine D, barretoxin, and swertiamarin; it has stable quality control, It is safe and effective, natural and non-toxic, and suitable for long-term use.
Owner:王双喜
Cyclovirobuxine-D sustained-release micro-pill capsules and preparation method thereof
ActiveCN101491509AEasy to operateHigh yieldOrganic active ingredientsAerosol deliveryDibutyl sebacateAdhesive
The invention relates to a buxine slow-release micro-pill capsule and a method for preparing the same. A slow-release micro-pill consisting of a hollow pill core, a main drug layer using Cyclovirobuxine D as an active ingredient and slow-release preparation auxiliary material layer is encapsulated into a capsule to prepare the buxine slow-release micropill capsule; the hollow pill core, the Cyclovirobuxine D as a main drug and the slow-release preparation auxiliary materials comprise the following weight percent: 70 to 95 percent of the hollow pill core, 1 to 5 percent of the Cyclovirobuxine D and the 4 to 25 percent of the slow-release preparation auxiliary materials; and the slow-release preparation auxiliary materials comprise acrylic resin as an adhesive, ethyl cellulose as a coating material, dibutyl sebacate as a plasticizer and talcum powder as a lubricant for encapsulation. The method has good repeatability; and the buxine slow-release micro-pill capsule has even products, good slow release effect and high bioavailability.
Owner:HANGZHOU CONBA PHARMA
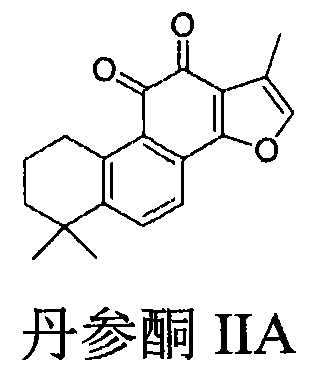
Preparation of compound with tanshinone IIA and cyclovirobuxine D composite structure and application of compound in prevention of cardiovascular diseases
ActiveCN106146605AGood water solubilityReduce usageOrganic active ingredientsSteroidsSolubilityTanshinone IIA
The invention relates to preparation of a compound with a tanshinone IIA and cyclovirobuxine D composite structure and an application of the compound in prevention of cardiovascular diseases. The compound is shown in a formula (I), wherein definition of X is shown in the specification. The compound shown in the formula (I) combines the main active ingredient tanshinone IIA of a traditional Chinese medicine danshen root and the main active ingredient cyclovirobuxine D of a traditional Chinese medicine Huangyangning, and according to modern molecular drug design, and effects of the tanshinone IIA and the cyclovirobuxine D for preventing and treating the cardiovascular diseases are retained while the defects of low solubility of the tanshinone IIA and insufficient purity of the cyclovirobuxine D as the main active ingredient in the traditional Chinese medicine Huangyangning are overcome. The compound shown in the formula (I) can be taken as a potential drug for preventing and treating the cardiovascular diseases.
Owner:北京桦冠医药科技有限公司
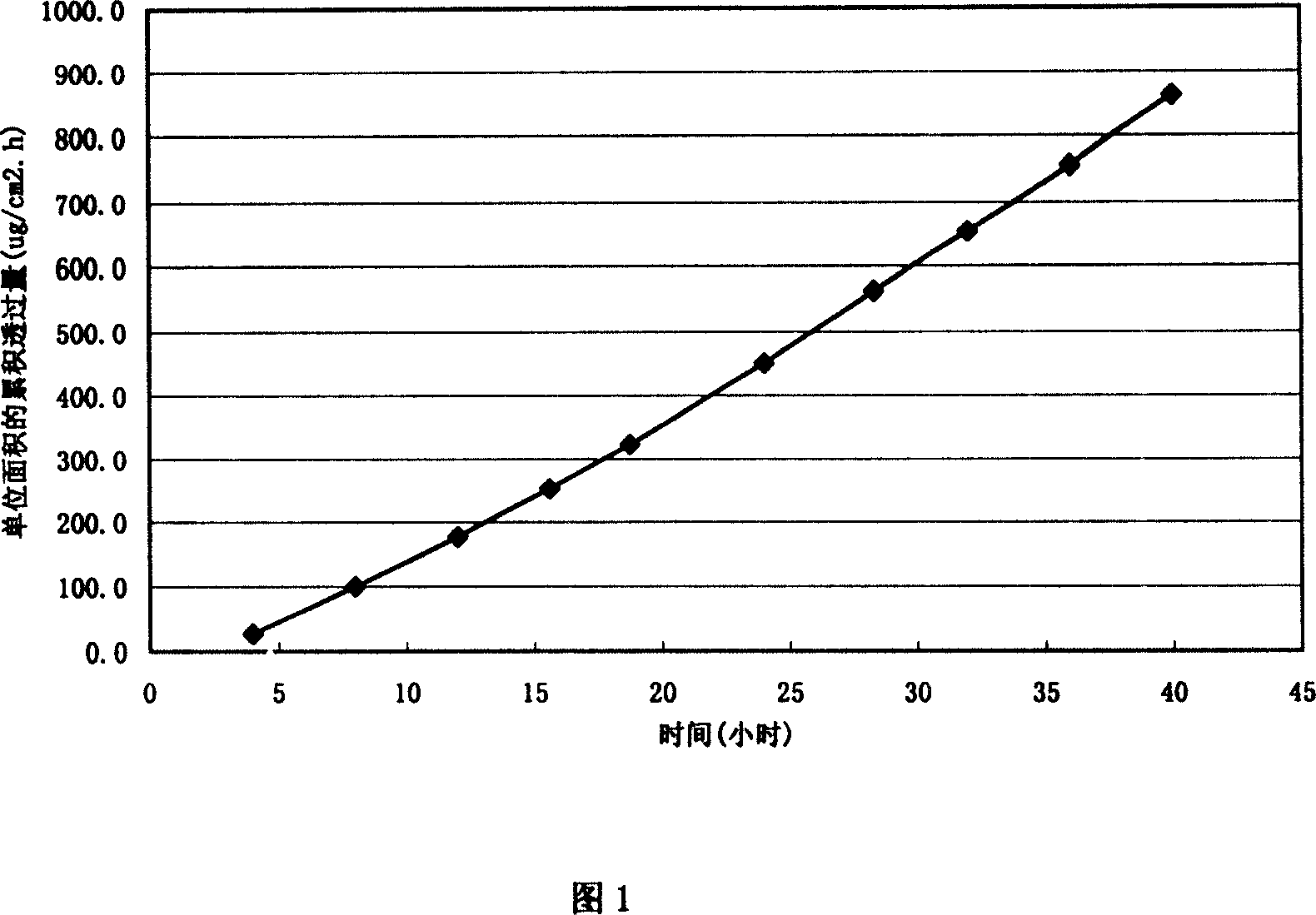
Plaster containing Chinese medicine cyclovirobuxine D and its preparing method
InactiveCN1985834ADrug safetyMedication safetyOrganic active ingredientsMacromolecular non-active ingredientsWater insolubleMedicine
The present invention discloses a kind of plaster containing Chinese medicine cyclovirobuxiine D and its preparation process. The plaster consists of a protecting layer, a medicine storing layer, a medicine-contact adhesive layer, a release controlling film layer and a lining layer. The medicine storing layer consists of cyclovirobuxiine D 15-50 wt% and water soluble matrix and / or water insoluble matrix 50-85 wt%; and the medicine-contact adhesive layer consists of cyclovirobuxiine D 2-10 wt%, water soluble matrix and / or water insoluble matrix 85-95 wt% and transdermal promoter 2-6 wt%. The plaster has clinically required transdermal speed and amount, determined curative effect and clinical safety.
Owner:TIANJIN ZHONGBAO PHARMACY
Traditional Chinese medicine raw material as well as preparation and use thereof
The invention belongs to the technical field of traditional Chinese medicines and discloses a traditional Chinese medicine raw material as well as a preparation and a use thereof, wherein the pharmaceutical raw material comprises cyclovirobuxine D and cycloprotobuxamine C. Studies find that the content of the cycloprotobuxamine C in the cyclovirobuxine D raw material is within a certain range, and the kidney toxicity of the raw material is significantly reduced; and pharmacodynamic tests prove that the cyclovirobuxine D pharmaceutical raw material disclosed by the invention has great effects of treating angina, coronary heart disease, arrhythmia and the like.
Owner:西藏易明西雅医药科技股份有限公司
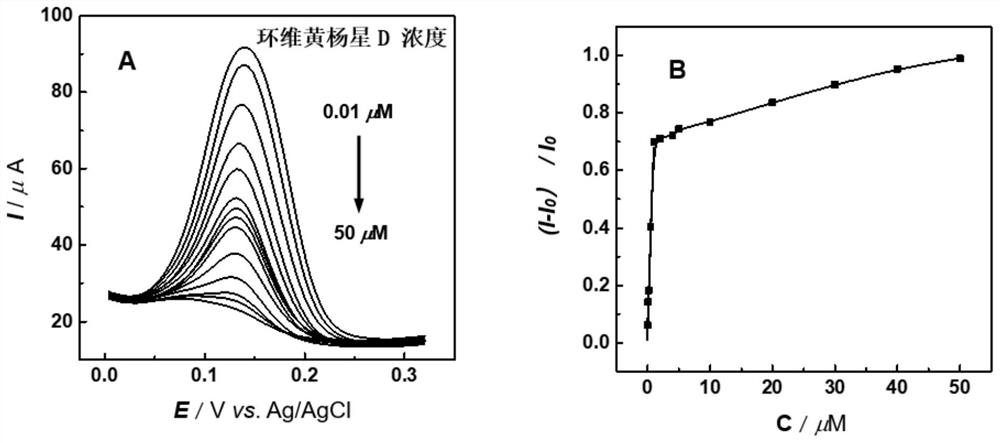
Electrochemical sensor, device and method for detecting cyclovirobuxine D
ActiveCN112179971AGood electron transport propertiesLarge specific surface areaMaterial electrochemical variablesBromothymol blueCarbon nanotube
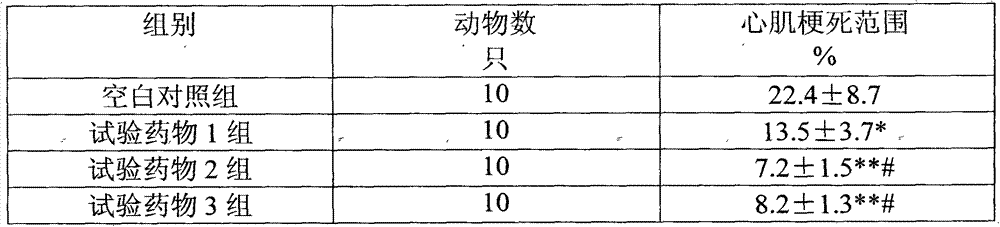
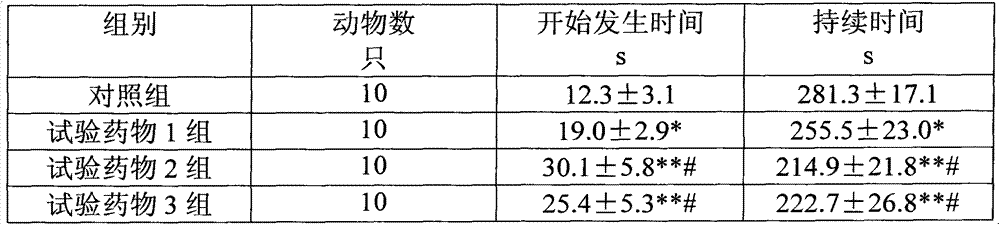
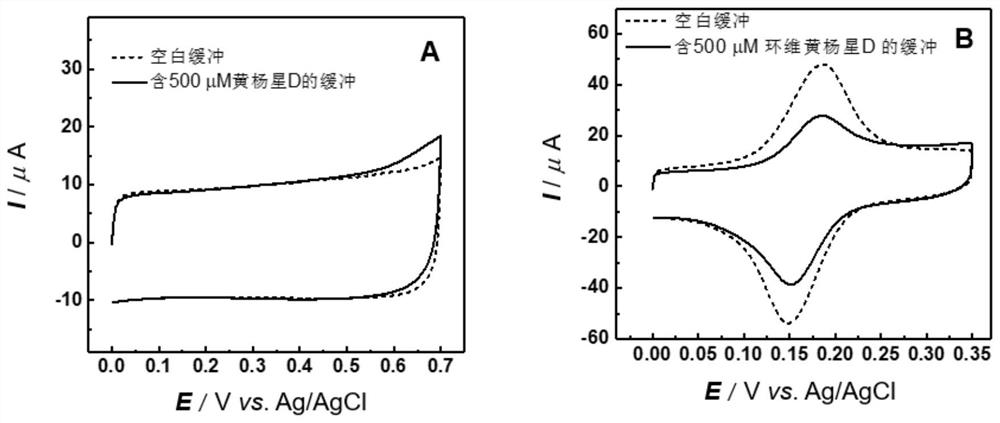
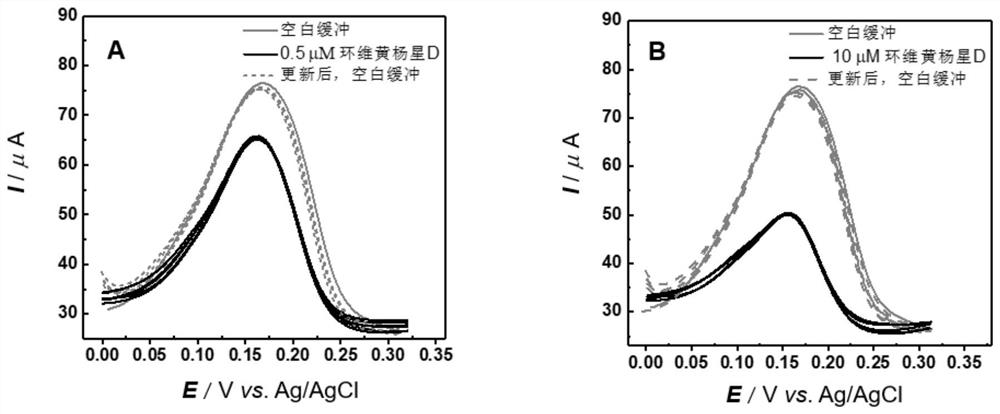
The invention relates to an electrochemical sensor, device and method for detecting cyclovirobuxine D. The electrochemical sensor comprises a glassy carbon electrode, a single-walled carbon nanotube is modified on the surface of the glassy carbon electrode, and polybromothymol blue is modified on the single-walled carbon nanotube. According to the invention, polybromothymol blue is used as a recognition element and an electrochemical probe of cyclovirobuxine D at the same time to construct the electrochemical sensor of cyclovirobuxine D, and quantitative analysis can be carried out on cyclovirobuxine D through the current change of the electrochemical sensor before and after the action with cyclovirobuxine D. The electrochemical sensor and the device are simple in preparation process, lowin cost, convenient to operate, high in analysis speed, sensitive in response, easy to update and reusable, and quantitative analysis of cyclovirobuxine D in various physiological samples can be realized. The electrochemical sensor, device and method provided by the invention have a wide application prospect in quality standard research of the cyclovirobuxine D and physiological and pathological events related to the cyclovirobuxine D.
Owner:ANHUI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Cyclovirobuxine D raw medicine and method for determining its content in preparation by chromatography
InactiveCN1786706BAccurate determination of true contentStrong specificityComponent separationFormateSilanes
The invention discloses a Cyclovirobuxine-D raw material drug and high efficiency liquid chromatography mensuration for Cyclovirobuxine-D content in different kinds of preparation, and high efficiency liquid chromatography for impurity testing in Cyclovirobuxine-D. The fixed phase in chromatographic condition uses octadecyl silane linkage silica gel or octyl silane linkage silica gel as filling material; The mobile phase adopt formate (ammonium formate) damping fluid-methanol system, acetic acid (ammonium acetate) damping fluid-methanol system, formate (ammonium formate) damping fluid-acetonitrile system or acetic acid (ammonium acetate) damping fluid-acetonitrile system; Evaporation light scattering detector (ELSD) detects drift tube temperature; drift tube temperature: 20-150 degree centigrade;and gas flow rate: 0.1L / min- 6.0L / min, split-flow or not split stream sampling. The method is easy to operate, timesaving, and has strong expert attribute. It could effectively divide some kind of alkaloids that has with Cyclovirobuxine-D in Cyclovirobuxine-D raw material drug. Thus, it is able to accurately measure their content. The method could also detecting impurity in Cyclovirobuxine-D. The invention could also measure content degree of homogeneity of Cyclovirobuxine-D in boxwood-nin piece, boxwood-nin drop pill, and boxwood-nin powder pin. And it commendably satisfies the request of pharmacopoeia content and content degree of homogeneity testing.
Owner:TIANJIN TASLY PHARMA CO LTD
Cyclovirobuxine D solid dispersion preparation method and novel medical application thereof
InactiveCN109078002AImprove apparent solubilityImprove in vitro dissolutionPowder deliveryOrganic active ingredientsSolubilityPharmacy
The invention relates to fields of chemical pharmacy, pharmaceutical preparations, pharmacokinetics and pharmacodynamics and discloses a solid dispersion prepared from cyclovirobuxine D extracted fromBuxus micr ophylla Sieb.et Zucc.var.sinica Rehd.et Wils. and congeners and application of the solid dispersion to prevention and treatment of senile dementia. According to enzymological and animal experiments, the cyclovirobuxine D solid dispersion has a great senile dementia prevention and treatment function. According to further researches, activity of the cyclovirobuxine D solid dispersion isremarkably improved as compared with that of cyclovirobuxine D. The cyclovirobuxine D solid dispersion has the advantage that the a preparation method is simple, convenient and easy in operation, thecyclovirobuxine D solubility and intestinal mucosa permeation rate are greatly increased, and accordingly bioavailability is improved.
Owner:JILIN ACAD OF TRADITIONAL CHINESE MEDICINE
Medical raw material as well as preparation and application thereof
The invention belongs to the technical field of traditional Chinese medicines and discloses a medical raw material as well as a preparation and an application thereof. The medical raw material comprises cyclovirobuxine D and cyclovirobuxine C. Through researches, people find that the content of cyclovirobuxine C in the raw material of cyclovirobuxine D is within a certain range, so that the hepatotoxicity of the raw material is remarkably reduced; and proved by pharmacodynamic tests, the raw material of cyclovirobuxine D disclosed by the invention has a favorable effect for treating angina, coronary disease and arrhythmia.
Owner:西藏易明西雅医药科技股份有限公司
A kind of Buyangning dispersible tablet and preparation method thereof
ActiveCN105476970BOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityDissolution
The invention discloses Huangyangning dispersible tablets and a preparation method thereof. Raw materials are subjected to nanocrystallization with a supercritical fluid method, then Cyclovirobuxine D, soybean lecithin, PEG4000, microcrystalline cellulose, hydroxypropyl cellulose, aerosil and aspartame are granulated with an equivalent incremental method by use of 60% ethyl alcohol, and drying and tableting are performed. Compared with Huangyangning on the market, the dispersible tablets have characteristics of high solubility and dissolution rate, quick absorption, high bioavailability and the like; the quality of the dispersible tablets can be better improved and development of drugs is facilitated.
Owner:INCREASE PHARMA YINGKOU CO LTD
Traditional Chinese medicine raw material as well as preparation and use thereof
The invention belongs to the technical field of traditional Chinese medicines and discloses a traditional Chinese medicine raw material as well as a preparation and a use thereof, wherein the pharmaceutical raw material comprises cyclovirobuxine D and cycloprotobuxamine C. Studies find that the content of the cycloprotobuxamine C in the cyclovirobuxine D raw material is within a certain range, and the kidney toxicity of the raw material is significantly reduced; and pharmacodynamic tests prove that the cyclovirobuxine D pharmaceutical raw material disclosed by the invention has great effects of treating angina, coronary heart disease, arrhythmia and the like.
Owner:西藏易明西雅医药科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com