Medical raw material as well as preparation and application thereof
A technology for pharmaceutical preparations and raw materials, applied in the field of medicine, can solve problems such as research on the composition of raw materials of Buxycin D without cyclovir
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
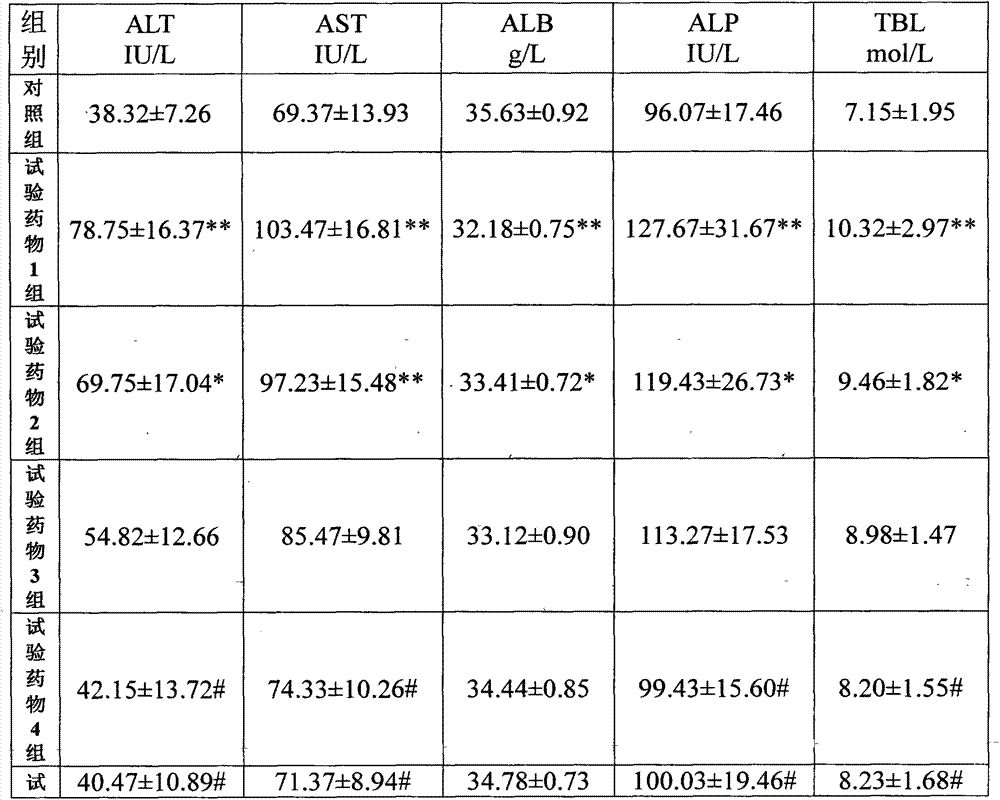
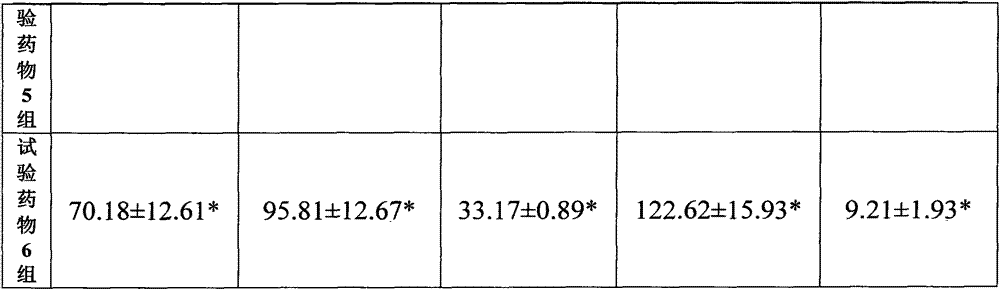
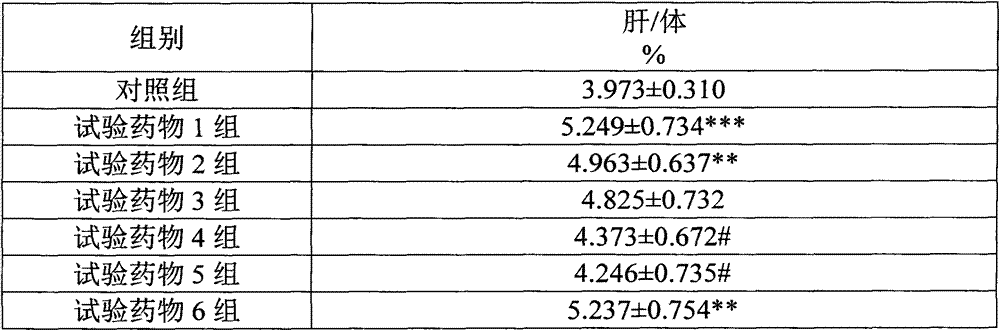
[0129] A pharmaceutical raw material, wherein the weight content of cyclovirbuxine D is 95.7%, the weight content of cycloevergreen boxine C is 3.9%, and the remaining impurities are 0.4%.
[0130] The above raw materials are prepared into aqueous injections, transfusions, powder injections, tablets, capsules and the like.
[0131] Wherein the aqueous injection is composed of: 1 g of Cycloviral Buxusin D raw material, 1000 ml of water for injection, an appropriate amount of tris-hydrochloric acid buffer solution (adjusting the pH value to 5.0); or 1 g of Cycloviral Buxusin D raw material, 1000 ml of water for injection, Appropriate amount of citric acid (adjust pH=5.0).
[0132]The composition of the powder injection: 1 g of cyclovir buxus D raw material, 1000 ml of water for injection, 50 g of mannitol, an appropriate amount of tris-hydrochloric acid buffer solution (adjust the pH value to 5.0); or 1 g of cyclovir buxuxin D raw material, manna Alcohol 50g, water for injectio...
Embodiment 2
[0134] A pharmaceutical raw material, wherein the weight content of cyclovirbuxine D is 99.1%, the weight content of cycloevergreen boxine C is 0.7%, and the remaining impurities are 0.2%.
[0135] The above raw materials are prepared into aqueous injections, transfusions, powder injections, tablets, capsules and the like.
[0136] Wherein the water injection is composed of: 2 g of cyclovir buxicine D raw material, 2000 ml of water for injection, an appropriate amount of tris-hydrochloric acid buffer solution (adjusting the pH value to 5.5); or 2 g of cyclovir buxicine D raw material, 2000 ml of water for injection, An appropriate amount of citric acid (adjust pH=5.5).
[0137] The composition of the powder injection: 2 g of cyclovir buxus D raw material, 2000 ml of water for injection, 50 g of mannitol, an appropriate amount of tris-hydrochloric acid buffer solution (adjust the pH value to 5.5); or 2 g of cyclovir buxuxin D raw material, manna Alcohol 50g, water for injectio...
Embodiment 3
[0139] A pharmaceutical raw material, wherein the weight content of cyclovirbuxine D is 99.3%, the weight content of cycloevergreen boxine C is 0.5%, and the remaining impurities are 0.2%.
[0140] The above raw materials are prepared into aqueous injections, transfusions, powder injections, tablets, capsules and the like.
[0141] Wherein the water injection is composed of: 2 g of cyclovir buxicine D raw material, 2000 ml of water for injection, an appropriate amount of tris-hydrochloric acid buffer solution (adjusting the pH value to 5.5); or 2 g of cyclovir buxicine D raw material, 2000 ml of water for injection, An appropriate amount of citric acid (adjust pH=5.5).
[0142] The composition of the powder injection: 2 g of cyclovir buxus D raw material, 2000 ml of water for injection, 50 g of mannitol, an appropriate amount of tris-hydrochloric acid buffer solution (adjust the pH value to 5.5); or 2 g of cyclovir buxuxin D raw material, manna Alcohol 50g, water for injectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com