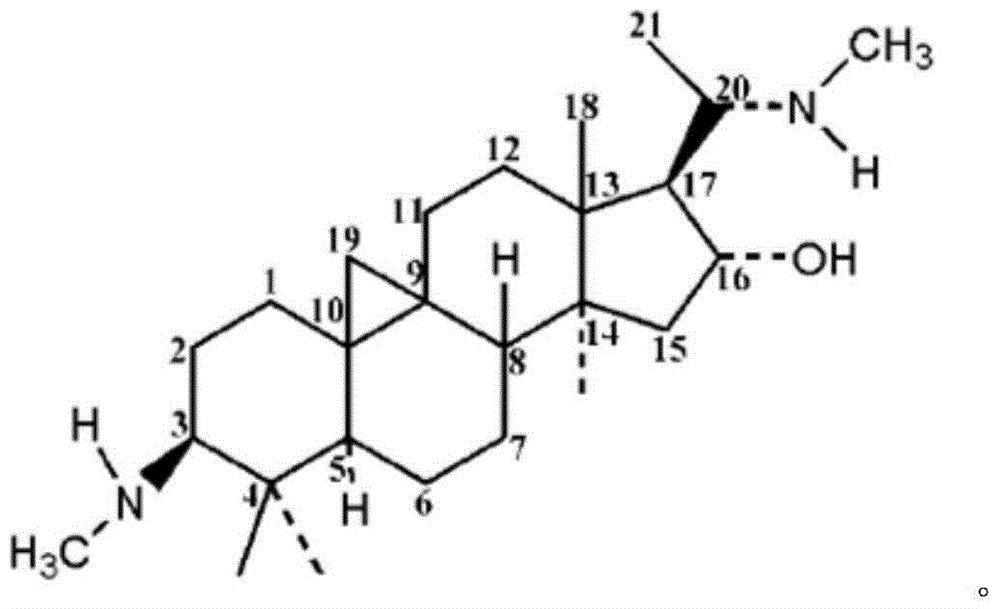
A kind of Buyangning dispersible tablet and preparation method thereof
A technology of dispersible tablets and Buyangning, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] 1) Use a supercritical reaction device to micronize Cyclovitamin D, wherein the machine parameters are: pre-expansion pressure 25MPA, pre-expansion temperature 320K, nozzle diameter 0.1mm, and obtain micronized Cyclovitamin D;
[0136] 2) Put 2 g of micronized cyclovir buxicine D and 3.7 g of soybean lecithin in an Erlenmeyer flask, use 200 ml of ethanol as the reaction solvent, heat and stir to 55 °C, and the rotation speed is 500 r / min, react for 1 h, and take the reaction solution The reaction solvent was removed by rotary evaporation at 60°C, and the solid was vacuum-dried for 1 hour to obtain the phospholipid complex;
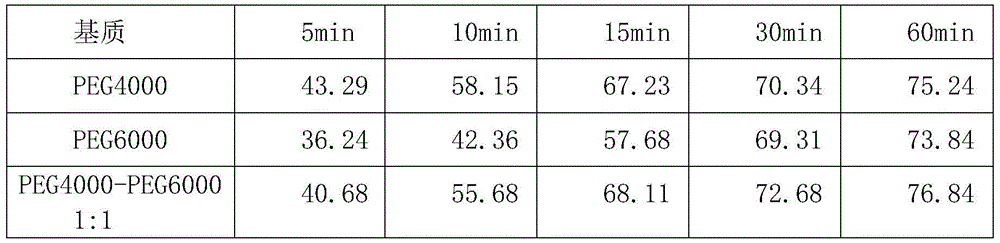
[0137] 3) Mix the phospholipid complex and PEG4000 according to the ratio of 1:2.5, after fully grinding evenly, spray a small amount of absolute ethanol, continue to grind into a uniform thick paste, dry in vacuum at 60°C for 3h, transfer to -20°C for curing for 24h , quickly pulverized after taking out, and passed through an 80-mesh sieve to obtai...
Embodiment 2
[0140] 1) Use a supercritical reaction device to micronize Cycloviral Buxillus D, wherein the machine parameters are: pre-expansion pressure 25MPA, pre-expansion temperature 370K, nozzle diameter 0.2mm, and obtain micronized Cycloviral Buxus D;
[0141] 2) Put 2 g of micronized cyclovir buxicine D and 3.7 g of soybean lecithin in an Erlenmeyer flask, use 200 ml of ethanol as the reaction solvent, heat and stir to 55 °C, and the rotation speed is 500 r / min, react for 1 h, and take the reaction solution The reaction solvent was removed by rotary evaporation at 60°C, and the solid was vacuum-dried for 1 hour to obtain the phospholipid complex;
[0142] 3) Mix the phospholipid complex and PEG4000 at a ratio of 1:2, grind them evenly, spray a small amount of absolute ethanol, continue to grind into a uniform thick paste, dry in vacuum at 60°C for 3 hours, transfer to -20°C and solidify for 24 hours , quickly pulverized after taking out, and passed through an 80-mesh sieve to obtain...
Embodiment 3
[0145] 1) Use a supercritical reaction device to micronize Cyclovitamin D, wherein the machine parameters are: pre-expansion pressure 35MPA, pre-expansion temperature 420K, nozzle diameter 0.5mm, and obtain micronized Cyclovitamin D;
[0146] 2) Put 2 g of micronized cyclovir buxicine D and 3.7 g of soybean lecithin in an Erlenmeyer flask, use 200 ml of ethanol as the reaction solvent, heat and stir to 55 °C, and the rotation speed is 500 r / min, react for 1 h, and take the reaction solution The reaction solvent was removed by rotary evaporation at 60°C, and the solid was vacuum-dried for 1 hour to obtain the phospholipid complex;
[0147] 3) Mix the phospholipid complex and PEG4000 at a ratio of 1:3. After fully grinding evenly, spray a small amount of absolute ethanol and continue grinding to form a uniform thick paste. Vacuum dry at 60°C for 3h, transfer to -20°C and solidify for 24h , quickly pulverized after taking out, and passed through an 80-mesh sieve to obtain a phosp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com