Method for determining content of cyclovirobuxine D
The technology of cyclovirobuxine and cyclovirobuxine is applied in the field of high-performance liquid chromatography determination method of pre-column derivatization, and can solve the problems of poor separation effect, undetermined appropriate dosage of derivatizing agent phenyl isocyanate, and the like, To achieve the effect of saving reagents, simple method and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 (crude drug: provided by Henan Longdu Pharmaceutical Factory)
[0035] Content determination was checked according to high performance liquid chromatography (Appendix VID of the Pharmacopoeia of the People's Republic of China in 2005).
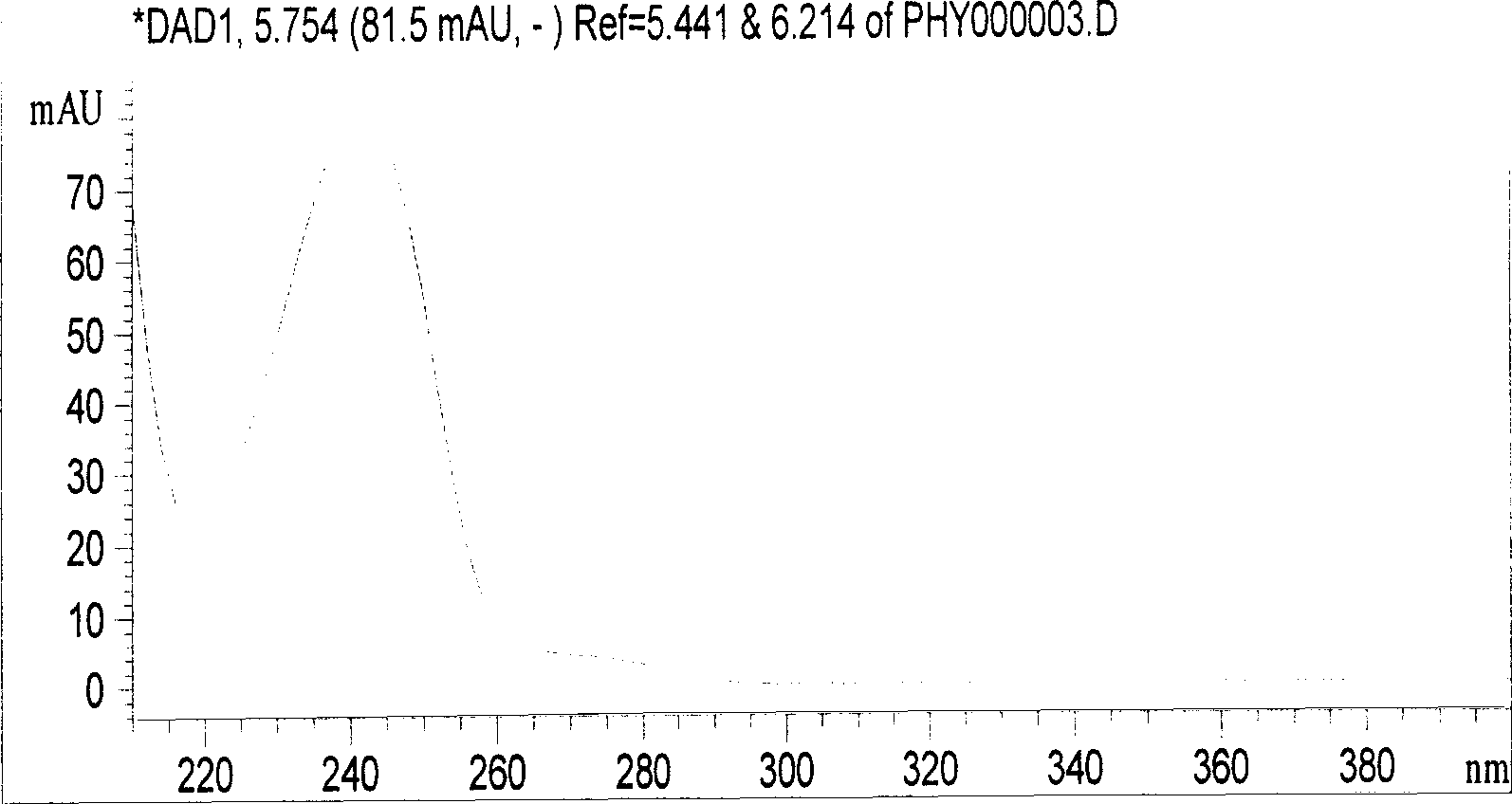
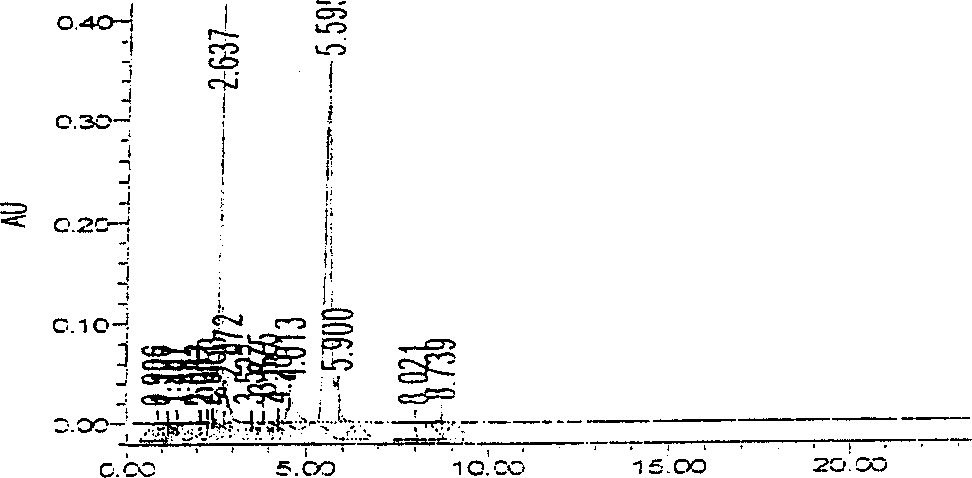
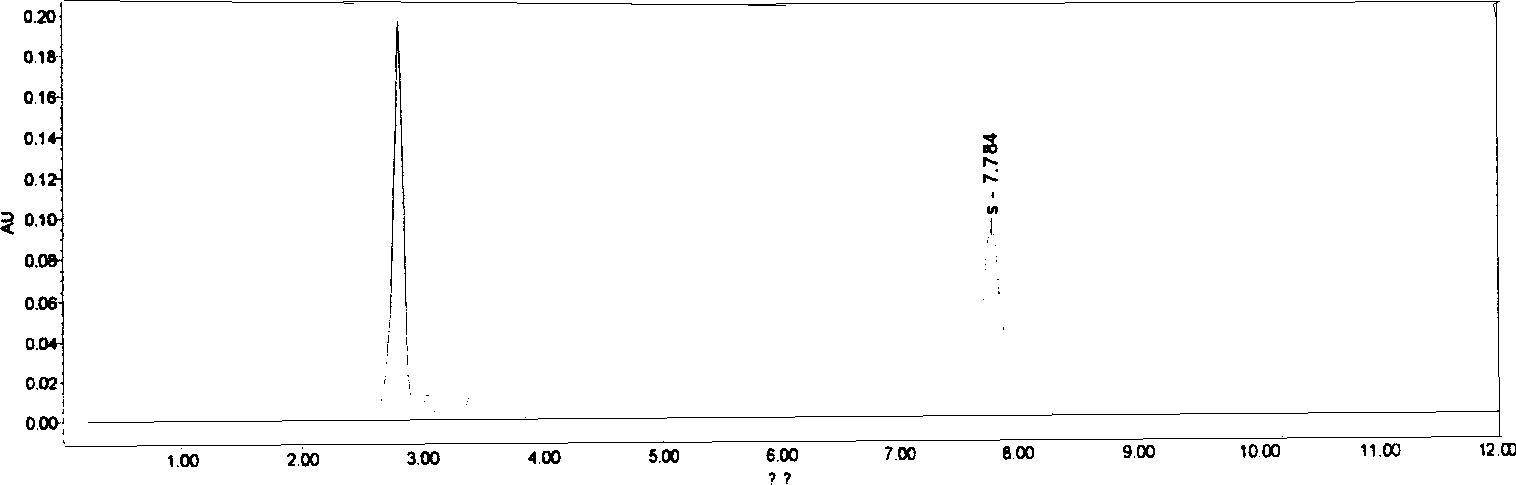
[0036] Chromatographic conditions and system adaptability Octadecylsilane bonded silica gel was used as filler; acetonitrile-water (volume ratio 70:30) was used as mobile phase; detection wavelength was 242nm. The number of theoretical boards should not be less than 5000 based on the calculation of the D peak of the ring-dimensional boxwood star.
[0037] Preparation of phenyl isocyanate test solution Precisely measure 0.1ml of phenyl isocyanate, put it in a 100ml measuring bottle, add chloroform to the mark, shake well, and you get it.
[0038] Preparation of Reference Substance Solution Accurately weigh an appropriate amount of cyclovirbuxine D reference substance dried at 105°C to constant weight, add chloroform to make...
Embodiment 2
[0041] Embodiment 2 (crude drug: provided by Jiangsu Dongtai Shentian Botanical Medicine Co., Ltd.)
[0042] Content determination was checked according to high performance liquid chromatography (Appendix VID of the Pharmacopoeia of the People's Republic of China in 2005).
[0043]Chromatographic conditions and system adaptability Octadecylsilane bonded silica gel was used as filler; acetonitrile-water (volume ratio 70:30) was used as mobile phase; detection wavelength was 242nm. The number of theoretical boards should not be less than 5000 based on the calculation of the D peak of the ring-dimensional boxwood star.
[0044] Preparation of phenyl isocyanate test solution Precisely measure 0.1ml of phenyl isocyanate, put it in a 100ml measuring bottle, add chloroform to the mark, shake well, and you get it.
[0045] Preparation of Reference Substance Solution Accurately weigh an appropriate amount of cyclovirbuxine D reference substance dried at 105°C to constant weight, add c...
Embodiment 3
[0048] Embodiment 3 (dropping pills: provided by Guangzhou Chenliji Pharmaceutical Factory, made by the patent No. 03126723.8 technical scheme)
[0049] Content determination was checked according to high performance liquid chromatography (Appendix VID of the Pharmacopoeia of the People's Republic of China in 2005).
[0050] Chromatographic conditions and system suitability test Octadecylsilane bonded silica gel was used as filler; acetonitrile-water (volume ratio 70:30) was used as mobile phase; detection wavelength was 242nm. The number of theoretical boards should not be less than 5000 based on the calculation of the D peak of the ring-dimensional boxwood star.
[0051] Preparation of phenyl isocyanate test solution Precisely measure 0.1ml of phenyl isocyanate, put it in a 100ml measuring bottle, add chloroform to the mark, shake well, and you get it.
[0052] Preparation of Reference Substance Solution Accurately weigh an appropriate amount of cyclovirbuxine D reference s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com