Buxine, buxine hydrochloride, and its preparing method and formulation
A technology of epiphylline hydrochloride and epiphylline, which is applied in pill delivery, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of high cost, and achieve the effects of low cost, clear extraction process route, and clear structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
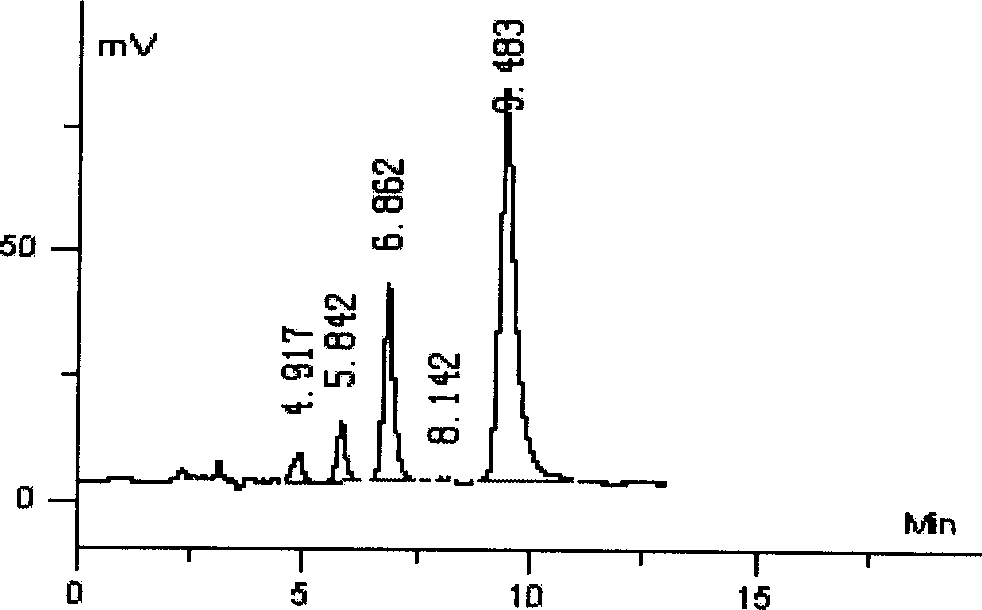
[0042] Preparation of boxicine: extract, separate and refine the effective part of alkaloids, boxicine, which contains three main alkaloid components, cycloviric boxwood D, cycloboxyline D and cycloevergreen boxicine C, from boxwood.
[0043] 1.1 Preparation of phylloxine
[0044] Boxwood, crushed into powder, soaked in 10 times the amount of ammonia solution (dilute concentrated ammonia water to 2 times the volume), soaked for 24 hours, added chloroform for extraction three times, combined the chloroform extracts, and recovered the chloroform under reduced pressure to obtain a residue. Add petroleum ether to disperse the residue, add dilute hydrochloric acid solution (3M) to extract four times, combine dilute hydrochloric acid solution, decolorize activated carbon, add concentrated ammonia water to neutralize dilute hydrochloric acid solution to alkaline (pH12), extract three times with chloroform, combine chloroform extract , most of the solvent was evaporated under reduced ...
Embodiment 2
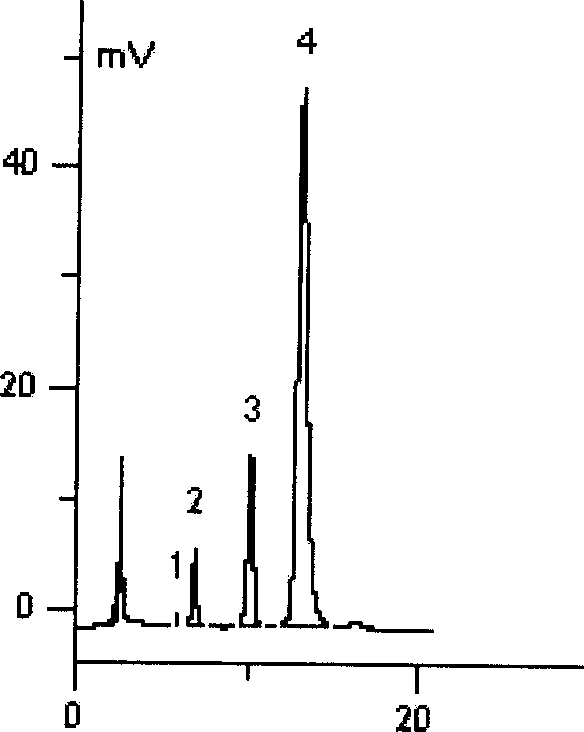
[0064] Embodiment 2: Epiphylline hydrochloride
[0065] 2.1 Preparation
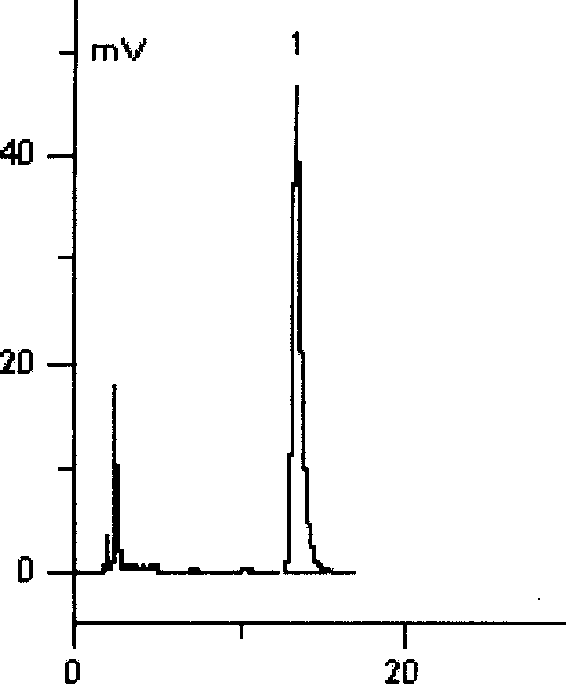
[0066] Get 2 grams of epiphylline (extracted by the method in Example 1), be dissolved in 50 milliliters of chloroform, pass into excessive dry hydrogen chloride gas in the chloroform solution under the stirring situation, press every mole of alkaloid (each composition) with 1 ~2 moles of hydrogen chloride are used to react with hydrogen chloride gas to form a precipitate of boxicine hydrochloride insoluble in chloroform. After the reaction is completed, filter out the precipitate, wash the precipitate twice with an appropriate amount of ethanol, and dry it under reduced pressure at 80°C. - Recrystallize from chloroform, dry under reduced pressure at 80°C to constant weight, and obtain boxyrine hydrochloride.
[0067] Solubility The solubility of boxicine hydrochloride in commonly used solvents is measured in Table 3. Compared with salicine, its solubility in water is significantly increased. The solu...
Embodiment 3
[0079] Embodiment 3: epiphylline injection
[0080] Injections made of epiphylline and / or its hydrochloride, including small needle solutions for injection, injections for intravenous infusion, sterile powders for injections, freeze-dried products, etc. The boxyline prepared in Example 1 was used for the boxyline, and the boxyline hydrochloride prepared in Example 2 was used for the boxylate hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com