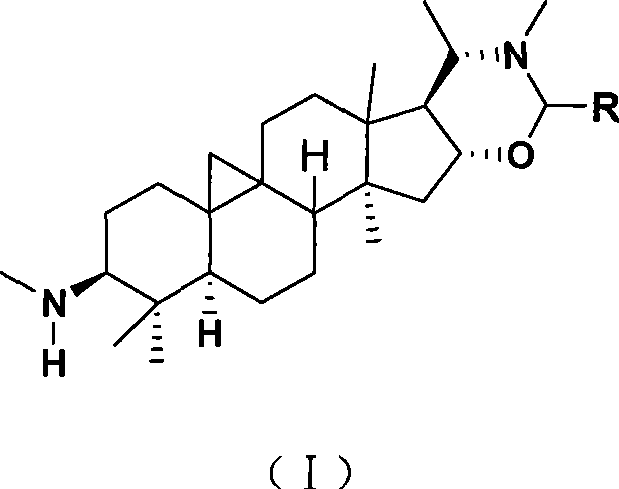
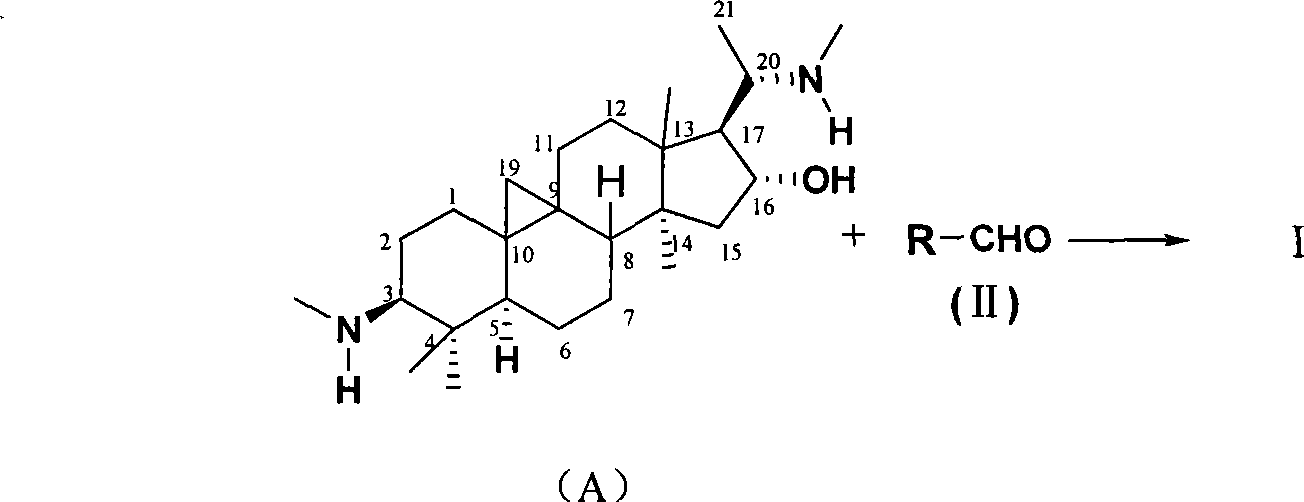
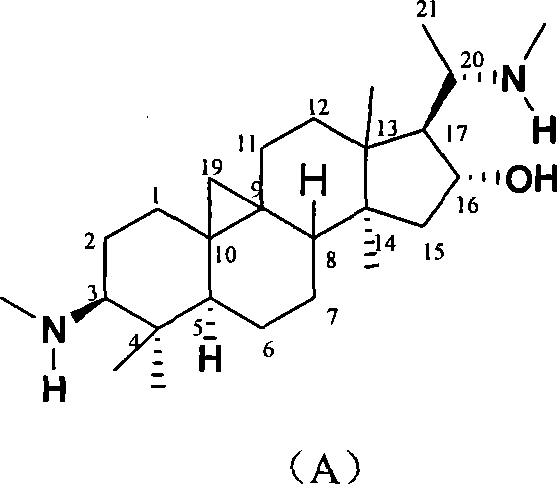
Cyclovirobuxine D derivative, and preparation and use thereof
A technology of cyclovirboxicin and its derivatives, which is applied in the field of cyclovirboxicin D derivatives and their preparation and application, can solve the problems of poor water solubility and narrow safety range of cyclovirboxicin D, and achieve low cost, The preparation method is simple and the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Take Cyclovirbuxine D (404mg) and p-nitrobenzaldehyde (155mg) in a 100mL round bottom flask, then add ethanol (40mL), stir and reflux for 1h; after the reaction is complete, recover the solvent under reduced pressure, recrystallize with ethanol, A yellow solid product (Ia) in which R in the structure of formula (I) is p-nitrophenyl was obtained, with a yield of 85%, m.p.228-230°C. Structural test results:
[0018] 1 HNMR (400MHz, CDCl 3 )δ: 7.67~8.21 (4H, dd, ArH), 5.10 (1H, s, OCHN), 3.89~3.93 (1H, septet, 16-H), 2.98~3.02 (1H, m, 20-H), 1.94 ~2.46(6H, m, 2NCH 3 ), 0.60~0.61 (1H, d, 19-βH), 0.34~0.35 (1H, d, 19-αH);
[0019] IR(KBr)ν: 3435, 2964, 2862, 1520, 1339, 1106, 989, 971, 856, 743;
[0020] HRMS (ESI) calcd for C 33 h 50 N 3 o 3 [M+H] + 536.3847, found 536.3855.
Embodiment 2
[0022] Using the same method as in Example 1, take Cyclovirubuxin D (404mg) and p-chlorobenzaldehyde (192mg) in a 100mL round bottom flask, then add isopropanol (40mL), stir and reflux for 4h; after the reaction, The solvent was recovered under reduced pressure, recrystallized with isopropanol alcohol, and the product (Ib) in which R in the structure of formula (I) was p-chlorophenyl was obtained, the yield was 82%, m.p.118-120°C. Structural test results:
[0023] 1HNMR (400MHz, CDCl3) δ: 7.29~7.43(4H, dd, ArH), 4.91(1H, s, OCHN), 3.85~3.89(1H, septet, 16-H), 2.87~2.90(1H, m, 20 -H), 1.96~2.46 (6H, m, 2NCH3), 0.59~0.60 (1H, d, 19-βH), 0.33~0.34 (1H, d, 19-αH);
[0024] IR (KBr) v: 3435, 2963, 2864, 1491, 1470, 1378, 1346, 1088, 970, 800, 722;
[0025] HRMS (ESI) calcd for C33H50ClN2O1 [M+H]+525.3606, found 525.3579.
Embodiment 3
[0027] Using the same method as in Example 1, take Cyclovirubuxin D (404mg) and benzaldehyde (140mg) in a 100mL round bottom flask, then add isopropanol (40mL), stir and reflux for 5h; after the reaction is complete, depressurize The solvent was recovered and recrystallized with isopropanol to obtain a white solid (Ic) in which R in the structure of formula (I) was phenyl, with a yield of 87%, m.p.194-196°C. Structural test results:
[0028] 1 HNMR (400MHz, CDCl 3 )δ: δ: 7.27~7.49(5H, m, ArH), 4.92(1H, s, OCHN), 3.87~3.92(1H, septet, 16-H), 2.85~2.90(1H, m, 20-H) , 1.99~2.46 (6H, m, 2NCH 3 ), 0.59~0.60 (1H, d, 19-βH), 0.33~0.34 (1H, d, 19-αH);
[0029] IR(KBr)ν: 3435, 2928, 2869, 1450, 1377, 1108, 990, 938, 742, 700;
[0030] HRMS (ESI) calcd for C 33 h 51 N 2 o 1 [M+H] + 491.3996, found 491.3980.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com