Cyclovirobuxine D solid dispersion preparation method and novel medical application thereof
A technology of Cyclovitamin and solid dispersion, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of low intestinal mucosal permeability and improve solubility and intestinal mucosal permeability, significant technological progress and innovation, and the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1 , the preparation of ring-dimensional buxicine D
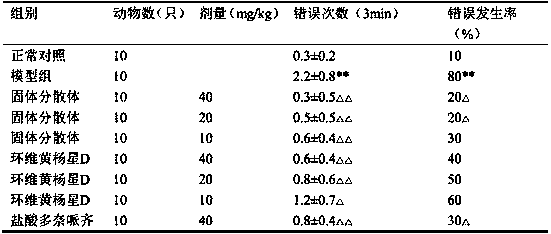
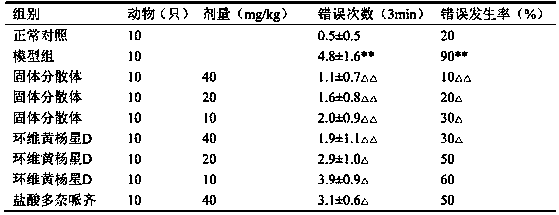
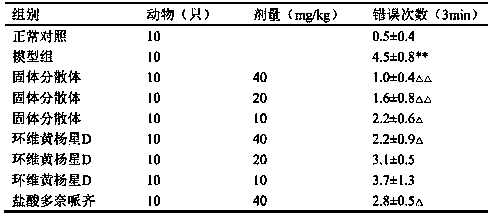
[0090] 5 kg of Euonymus microphylla was crushed into coarse powder, refluxed and extracted 3 times with 80% ethanol containing 2% glacial acetic acid, each time for 2 hours, combined the extracts, added 0.2% active carbon of the liquid volume, stirred, filtered, and the filtrate decompressed and recovered ethanol to obtain Extract, add water to dissolve, so that each 1ml contains 1g of medicinal materials, centrifuge, take the supernatant, pass through D001-CC macroporous strong acid cation exchange resin, first elute with 15% ethanol for 5 times the volume of the resin, and then adjust the pH with ammonia water Elute 5 times the volume of the resin with 70% ethanol of 11, collect the eluate of ammonia ethanol, recover the solvent, dry under reduced pressure, and obtain the composition with Cyclovirubuxin D as the main component. The content of cyclovirboxicin D in the composition is 83.5%. Take 1.0 g of the...
Embodiment 2
[0091] Example 2 , Preparation of Cycloviral Buxusine D Solid Dispersion
[0092] Take 10g of Cycloviral Buxillus D, 60g of PEG6000, and 5g of sodium caprate; dissolve Cycloviral Buxusin D in 500ml of ethanol, then add PEG6000 to dissolve completely, add the absorption-promoting agent sodium caprate, and then under reduced pressure The solvent was removed by rotary evaporation, dried by heating, pulverized and sieved.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com