A kind of ophthalmic compound preparation and preparation method thereof
A compound preparation and ophthalmic technology, which can be used in medical preparations without active ingredients, medical preparations containing active ingredients, and drug combinations, etc., and can solve the problems of poor adhesion, poor stability, and short storage time of active ingredients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Preparation of Carboxylated β-Cyclodextrin Crosslinked by Chondroitin Sulfate
[0075] 1) Dissolve 10mmol of monocarboxylated β-cyclodextrin (each molecule of cyclodextrin contains a single carboxylation group unit) and 12mmol of N-hydroxysuccinimide in 50mL of distilled water, add 12mmol of EDC, ice bath conditions Stir and react for 2 hours; then slowly add an aqueous solution of chondroitin sulfate with amino units (the content of chondroitin sulfate is 18 mmol), stir slowly for 1 hour in an ice bath, then raise the temperature to 35°C in a water bath, keep warm and slowly stir for 12 hours;
[0076] 2) After the reaction is completed, the reaction solution is concentrated, and the concentrated solution is dialyzed twice in a dialysis bag for 16 hours to remove unreacted small molecule raw materials, and then purified by silica gel column chromatography to obtain chondroitin sulfate-modified carboxylated β-ring Dextrin; the purification parameters are: silica gel col...
Embodiment 2
[0080] Preparation of Ophthalmic Compound Preparation 1
[0081] (1) Preparation of component A
[0082] S1-1: Weigh 3g of sodium hyaluronate and 2g of trehalose into 65ml of distilled water, stir and swell in a water bath at 80°C for 30min, fully dissolve and lower the temperature to room temperature; add 0.6g of betaine while stirring, and keep stirring until dissolved, then add 1g of alanylglutamine, continue to stir and dissolve, then add distilled water until the total weight of the solution is 100g, as the base solution;
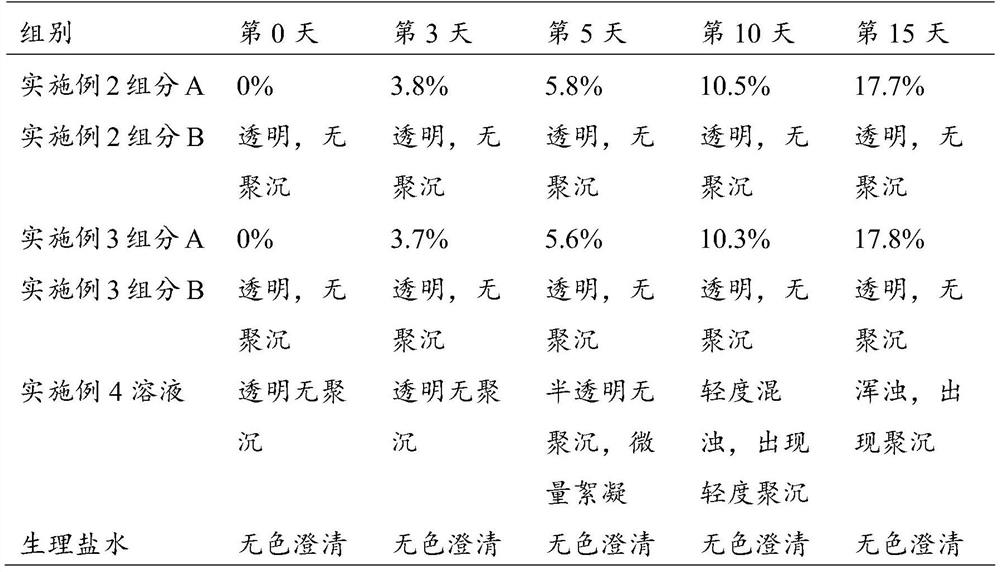
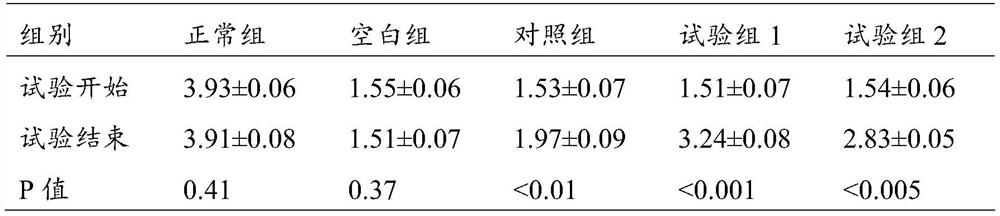
[0083] S1-2: Immerse 10 g of the chondroitin sulfate cross-linked modified cyclodextrin prepared above into the above base solution, heat it in a water bath to 45 °C, stir slowly for 30 min, and then treat it under 100 W ultrasonic for 3 min; stand at 4 °C for 6 h Afterwards, it was frozen at -80°C for 6 hours, and about 17 g of solid component A was obtained by vacuum freeze-drying.
[0084] After the obtained solid was sterilized by ultraviolet rad...
Embodiment 3
[0090] Preparation of ophthalmic compound preparation 2
[0091] (1) Preparation of component A
[0092]S1-1: Weigh 2g of sodium hyaluronate and 1.5g of trehalose into 60ml of distilled water, stir and swell in a water bath at 80°C for 30 minutes, and after fully dissolving, lower the temperature to room temperature; add 0.5g of betaine while stirring, and continuously Stir until dissolved, then add 1.2g of alanyl glutamine, continue to stir and dissolve, then add distilled water until the total weight of the solution is 100g, as the base liquid;
[0093] S1-2: Immerse 8 g of the cyclodextrin cross-linked and modified by chondroitin sulfate above into the above base solution, heat it in a water bath to 45°C, stir slowly for 30 minutes, and then treat it under 150W ultrasonic for 3 minutes; Freeze at -80°C for 6 hours, and obtain solid component A by vacuum freeze-drying.
[0094] After the obtained solid was sterilized by ultraviolet radiation, it was divided into sterilized...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com