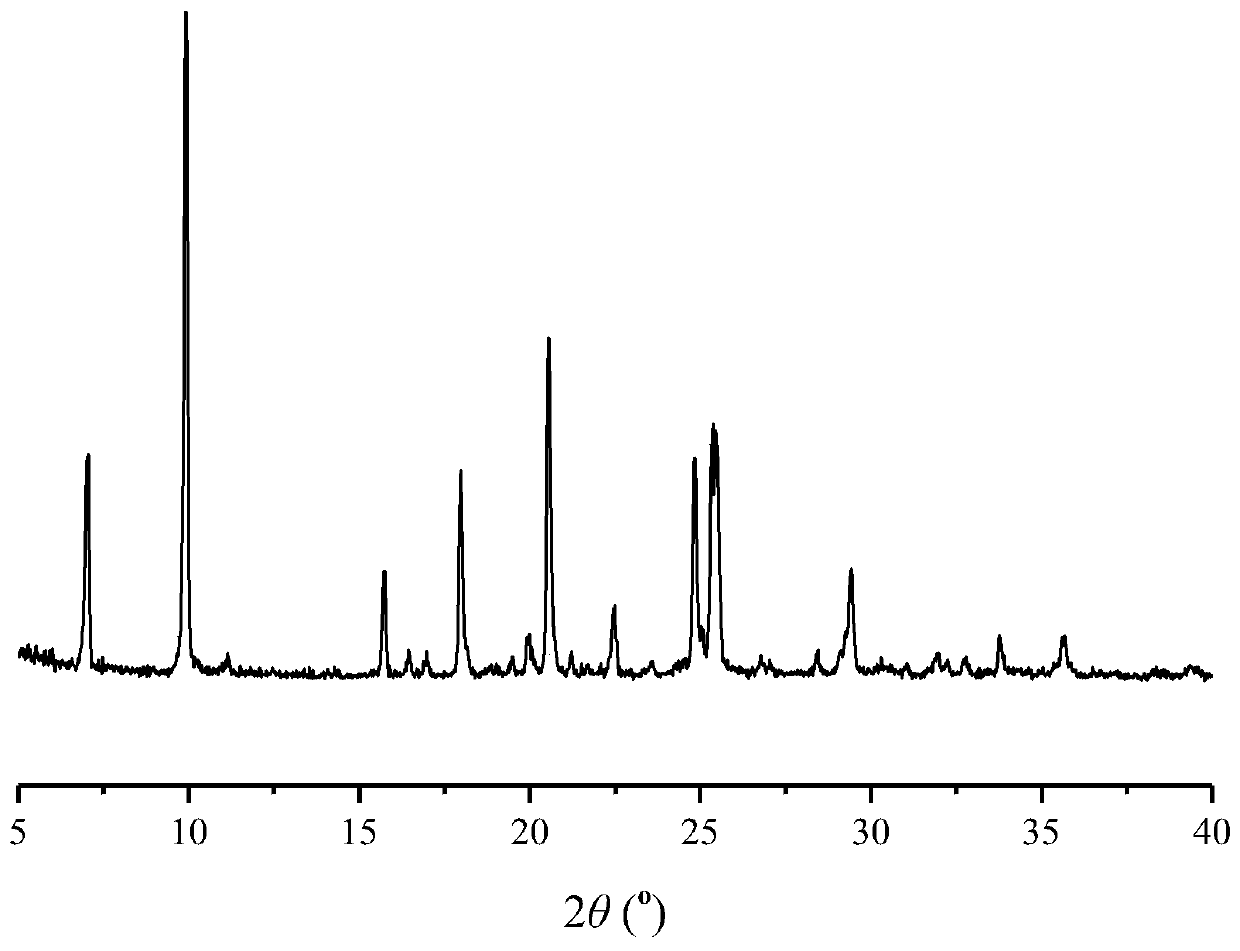
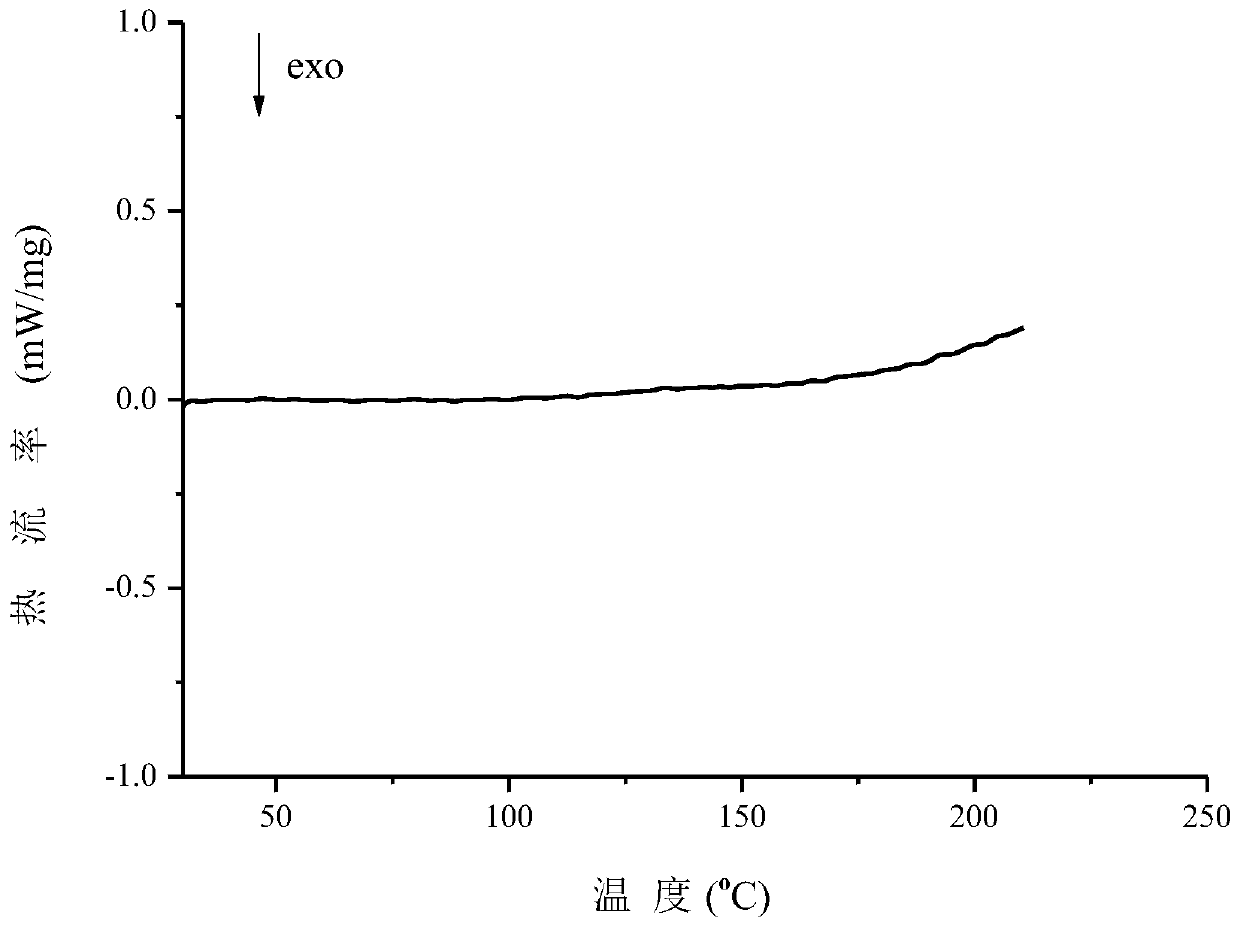
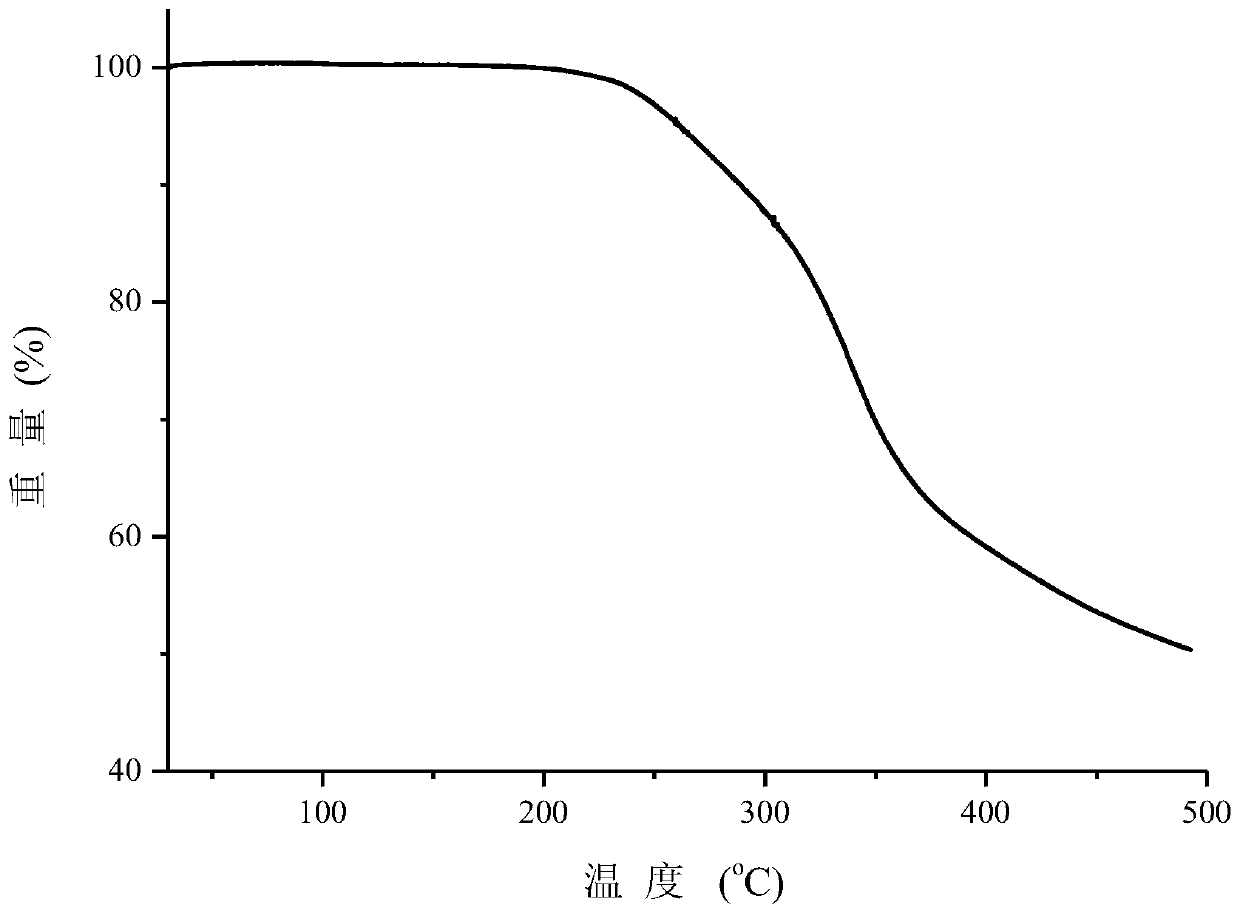
A co-crystal of penciclovir and 3,5-dihydroxybenzoic acid and its preparation method
A technology of dihydroxybenzoic acid and penciclovir, which is applied in the field of medicinal chemistry, can solve the problems of easy-to-cause phlebitis in clinical application, poor water solubility of penciclovir, and great irritation to human body, and achieve easy control of the crystallization process. Good performance and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]Weigh 63.3mg of penciclovir and 308.2mg of 3,5-dihydroxybenzoic acid, add 1mL of 50% ethanol (the volume ratio of ethanol and water is 1:1) to obtain a suspension, and place the suspension in Stir at room temperature for 24 hours, filter with suction, wash the resulting white solid with an appropriate amount of 50% ethanol, and then dry at 40°C for 48 hours to obtain a solid sample of penciclovir and 3,5-dihydroxybenzoic acid cocrystal.
Embodiment 2
[0045] Weighed 63.3 mg of penciclovir and 231.2 mg of 3,5-dihydroxybenzoic acid, added 1 mL of ethanol to obtain a suspension, stirred the suspension at room temperature for 2 h, and filtered it with suction. The resulting white solid was dried and obtained a spray Solid sample of co-crystal of ciclovir and 3,5-dihydroxybenzoic acid.
Embodiment 3
[0047] Weigh 63.3 mg of penciclovir and 231.2 mg of 3,5-dihydroxybenzoic acid, add 1 mL of methanol to obtain a suspension, stir the suspension at room temperature for 12 hours, filter with suction, and dry the obtained white solid to obtain Solid sample of co-crystal of penciclovir and 3,5-dihydroxybenzoic acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com