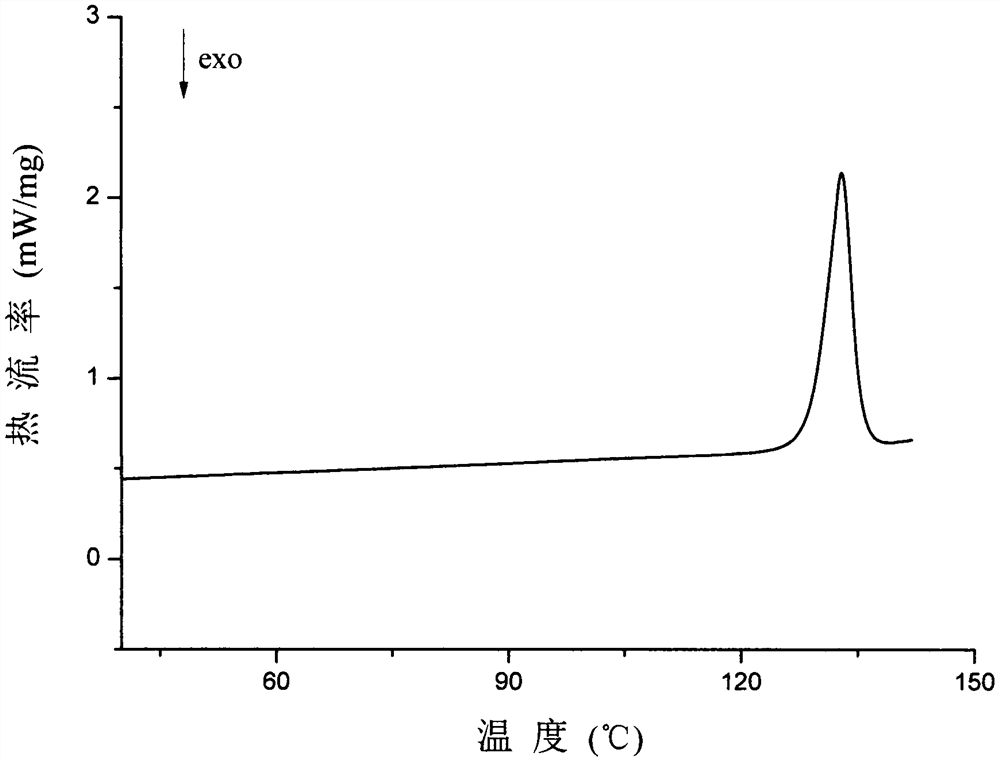
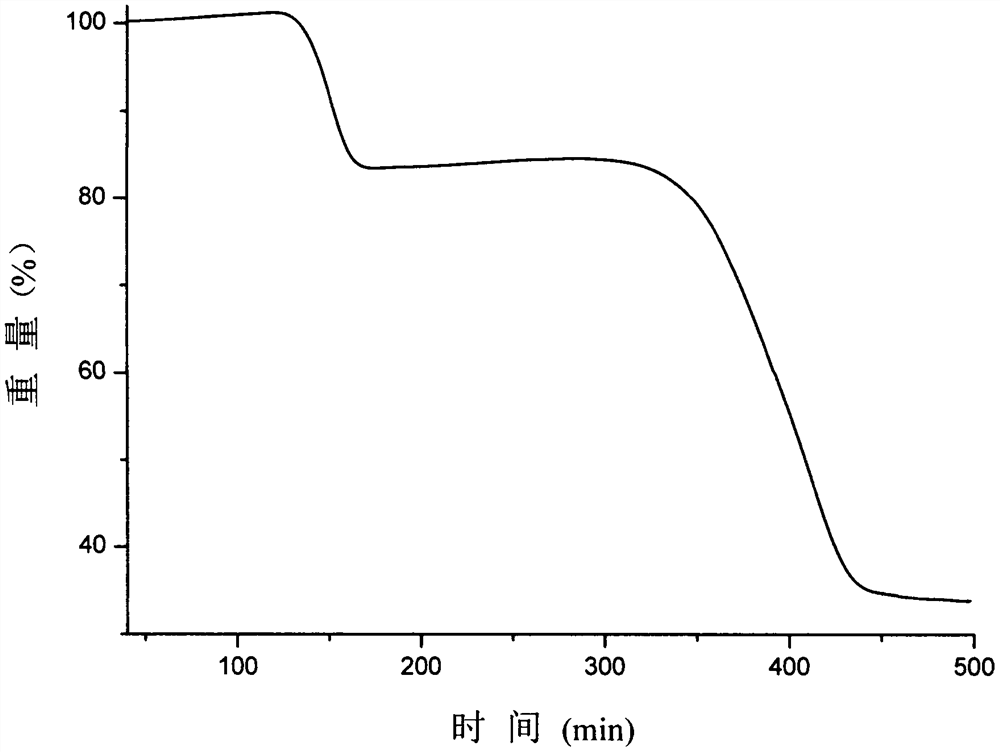
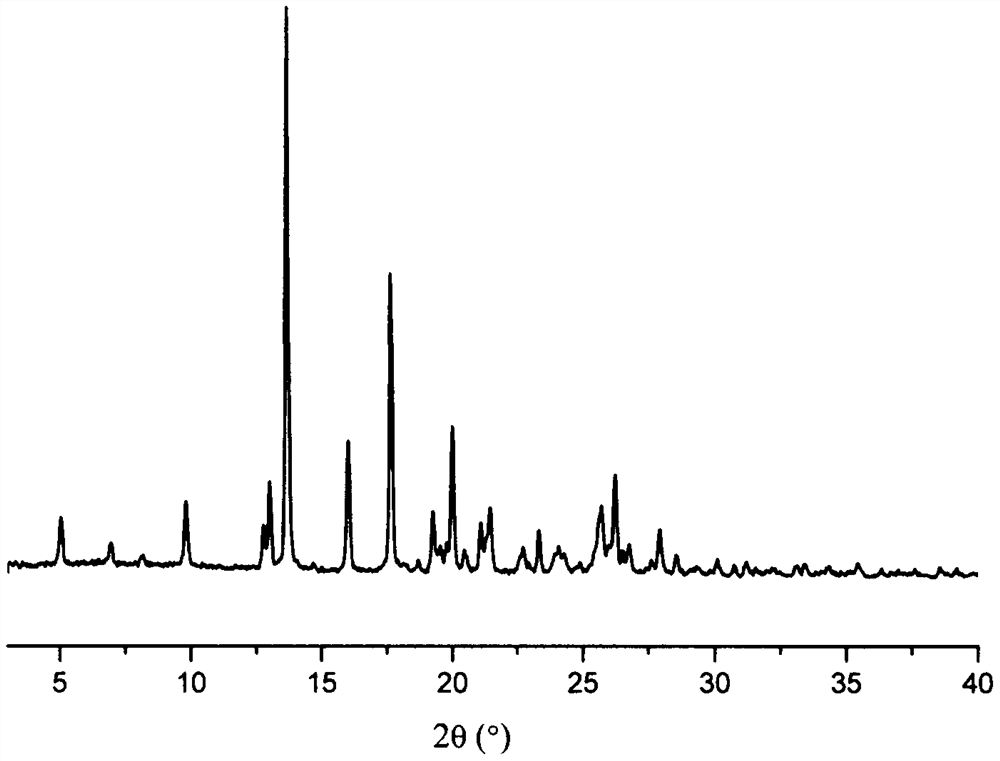
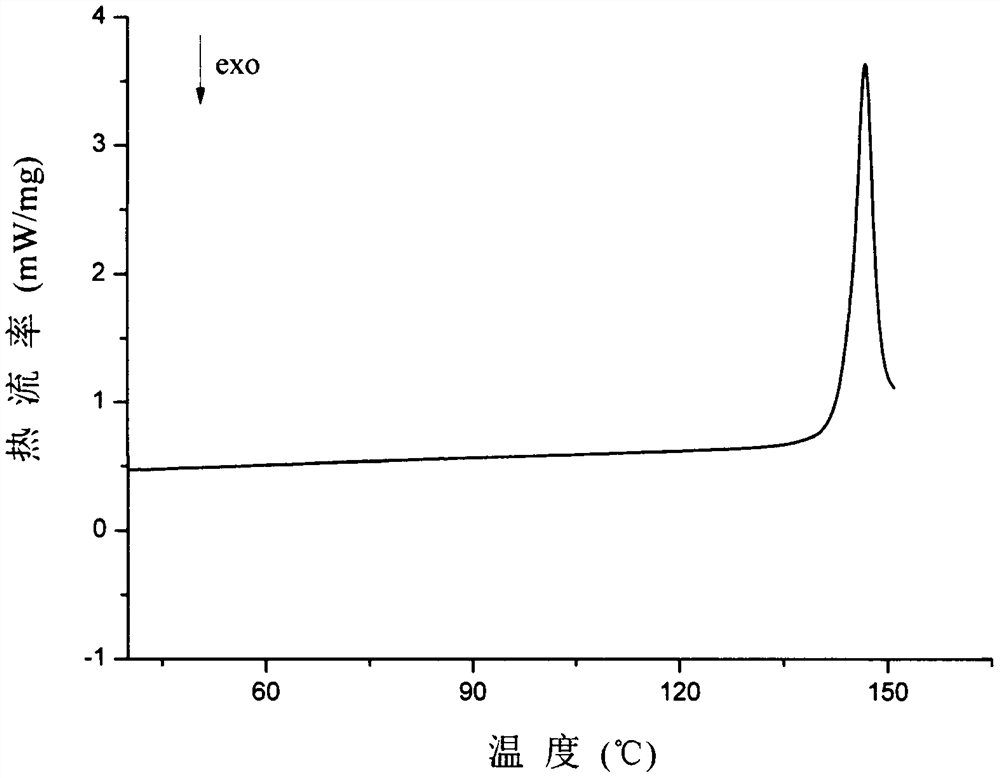
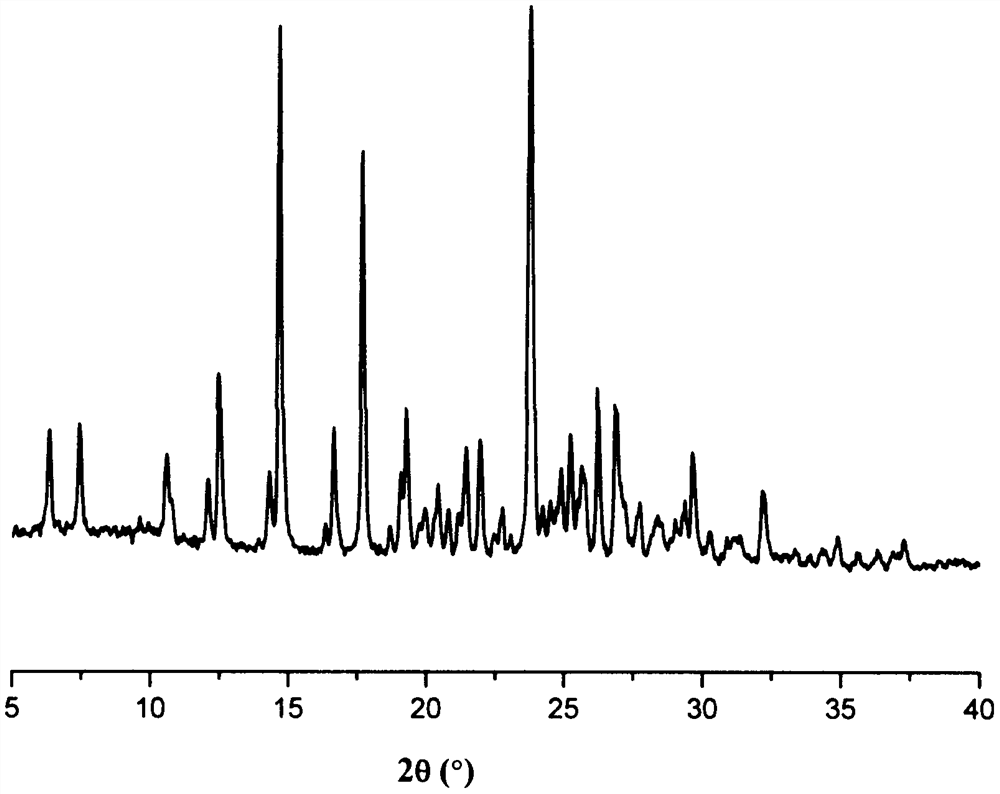
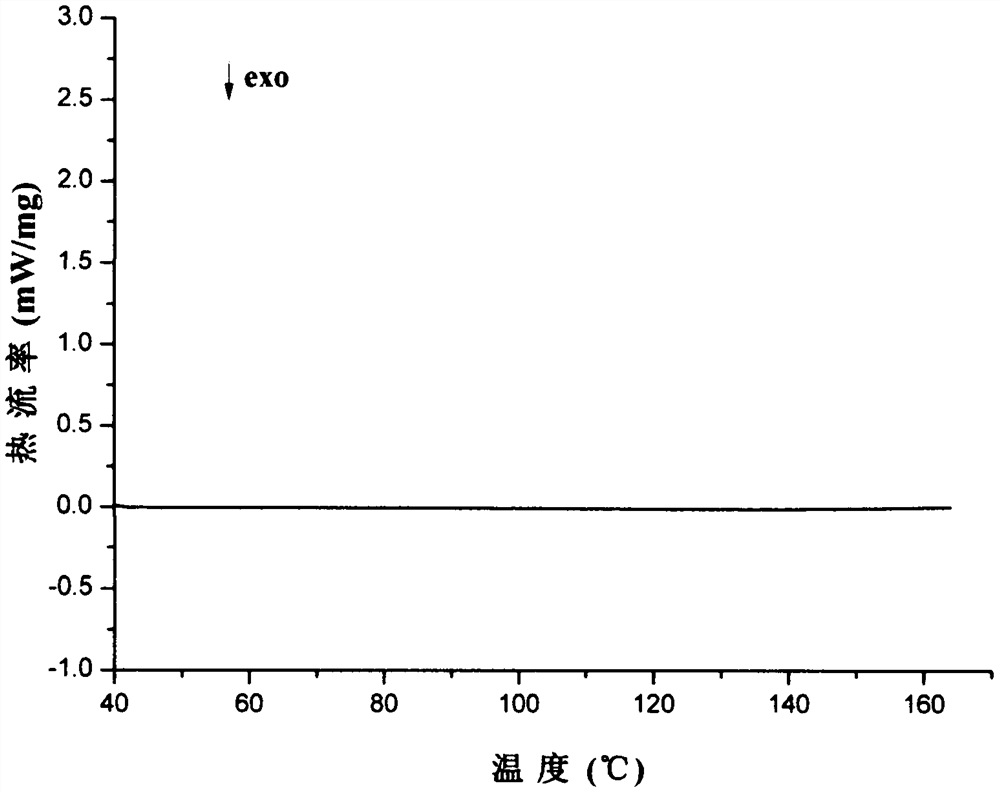
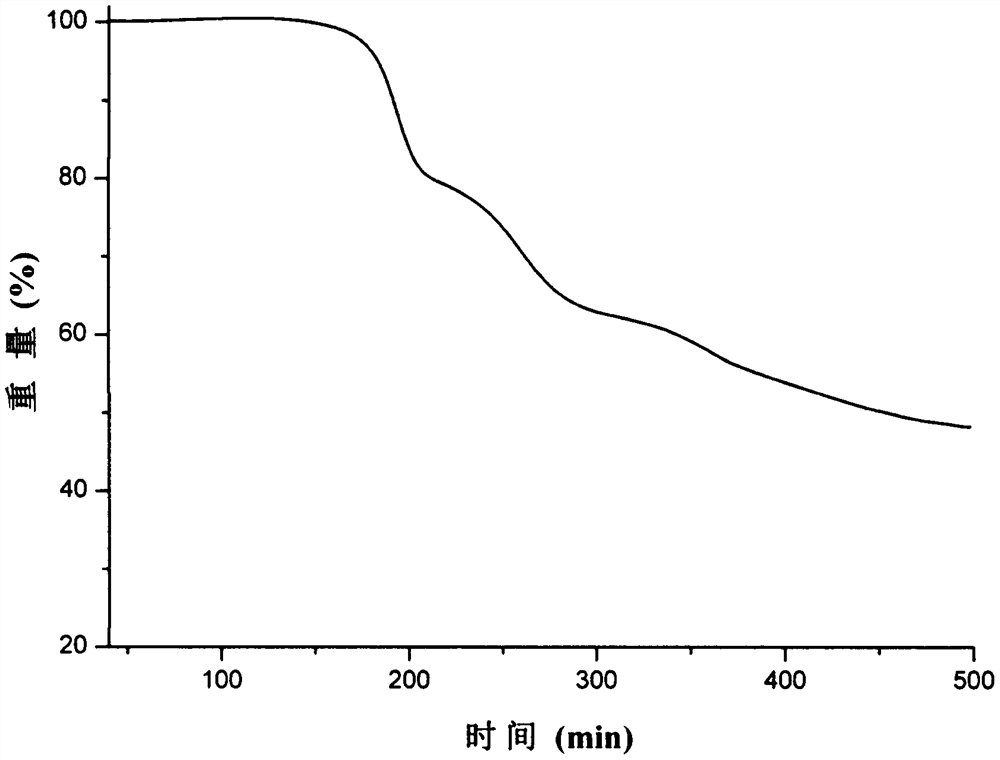
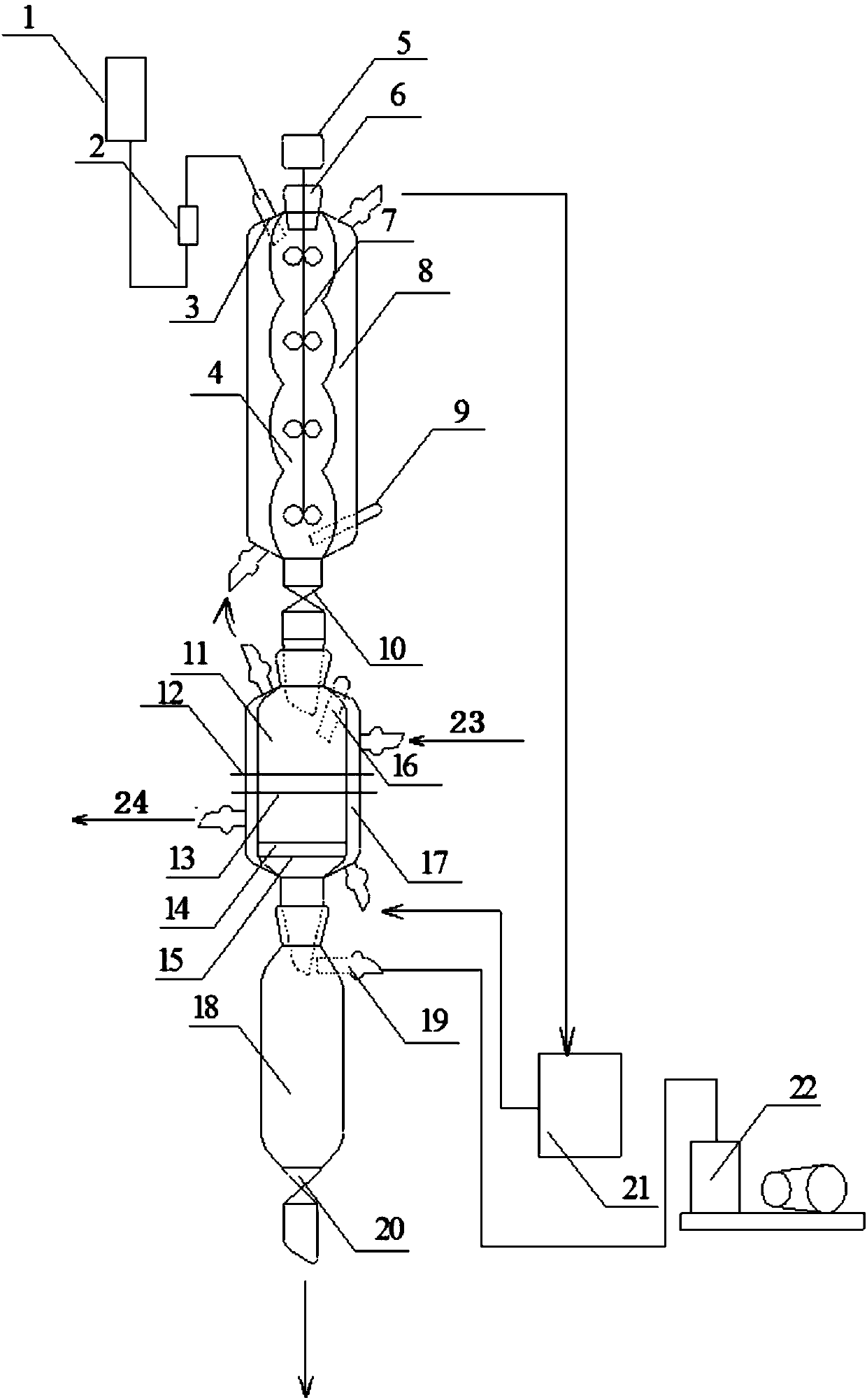
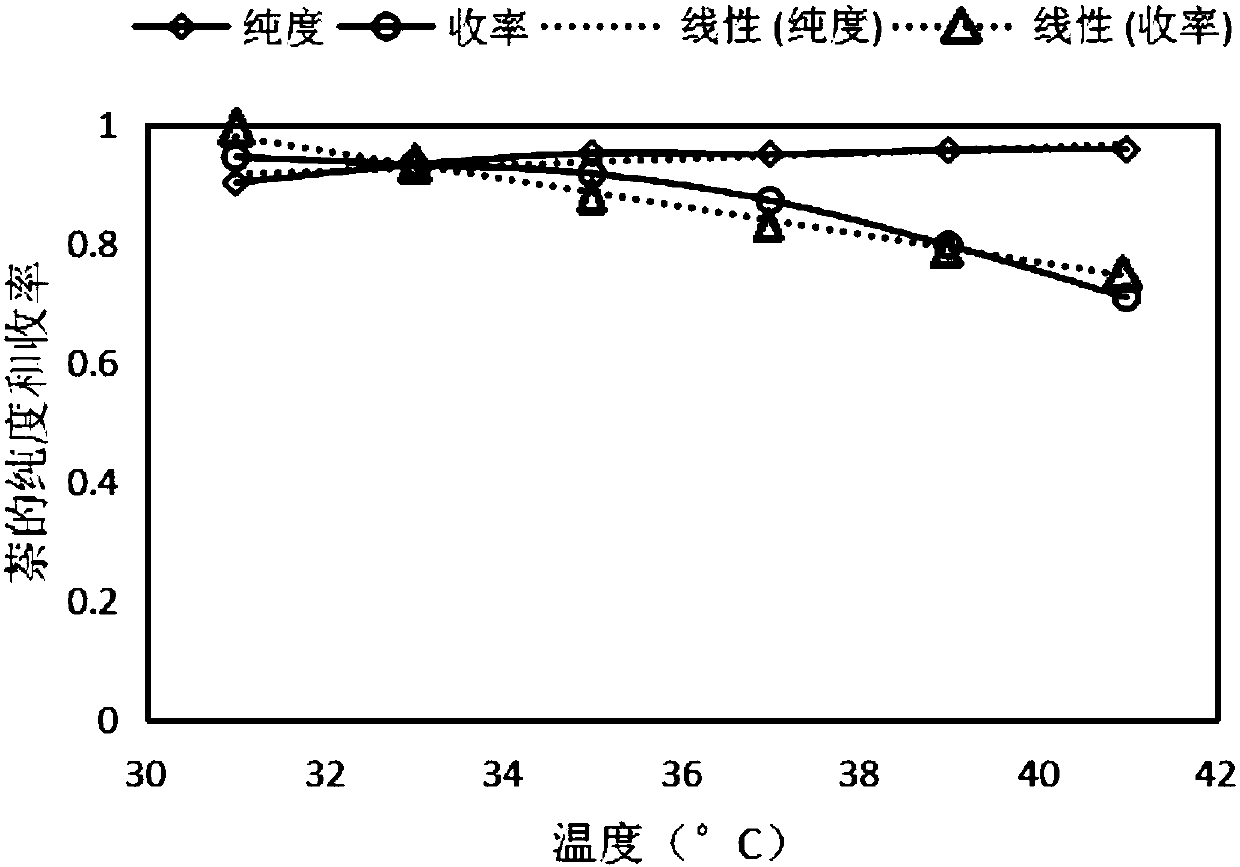
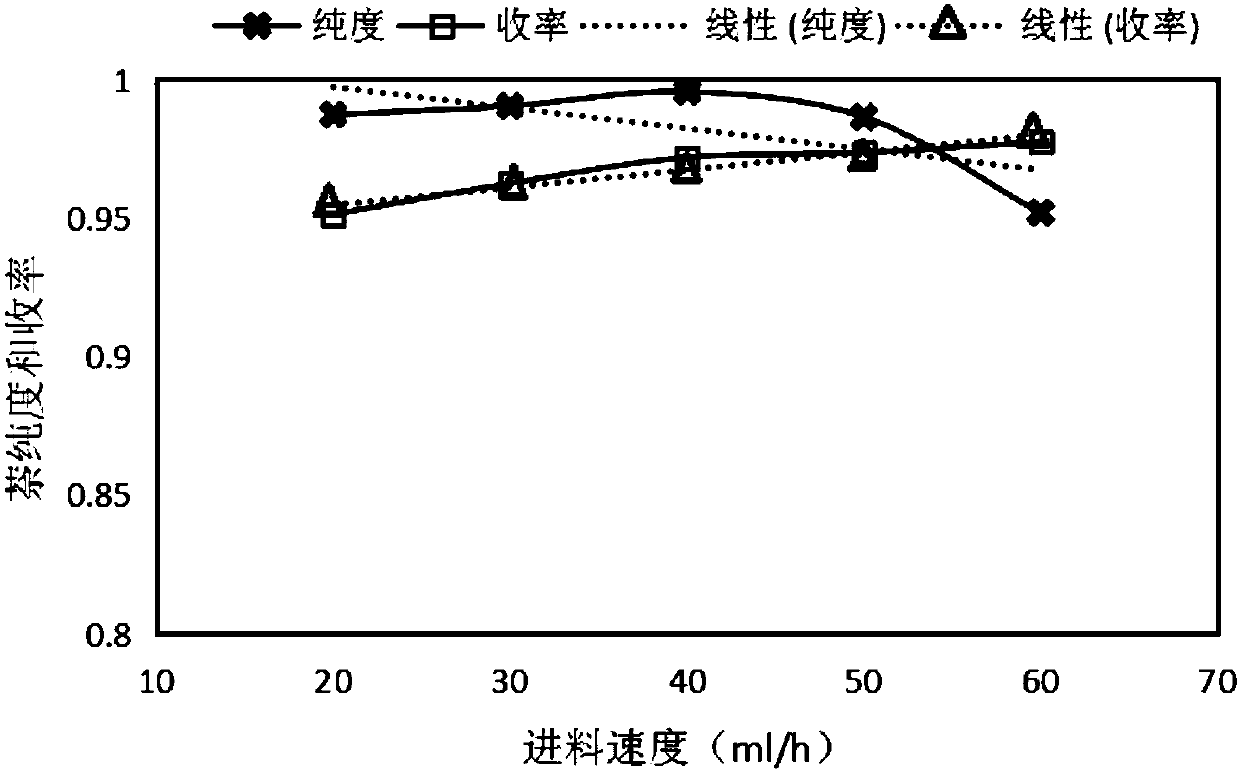
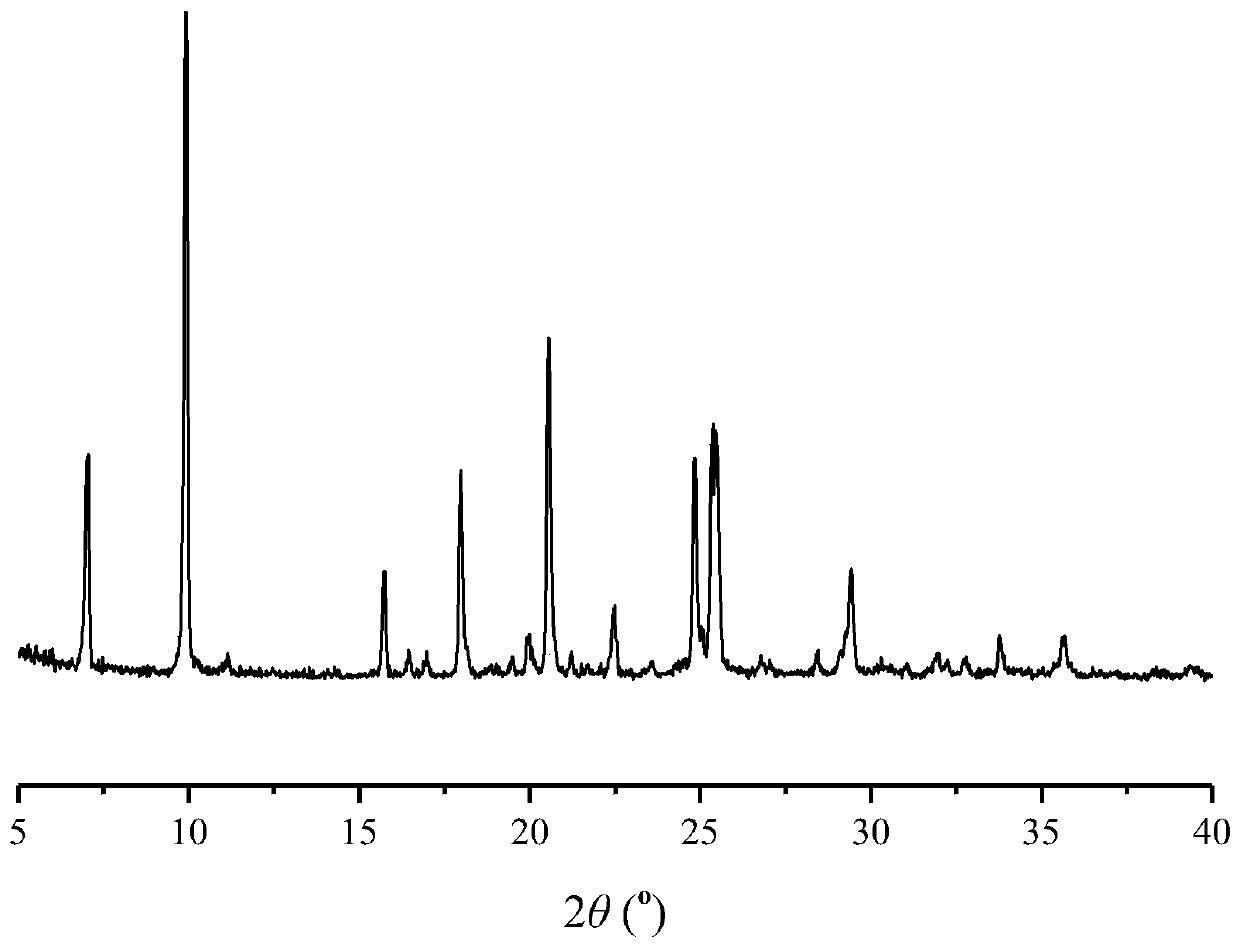
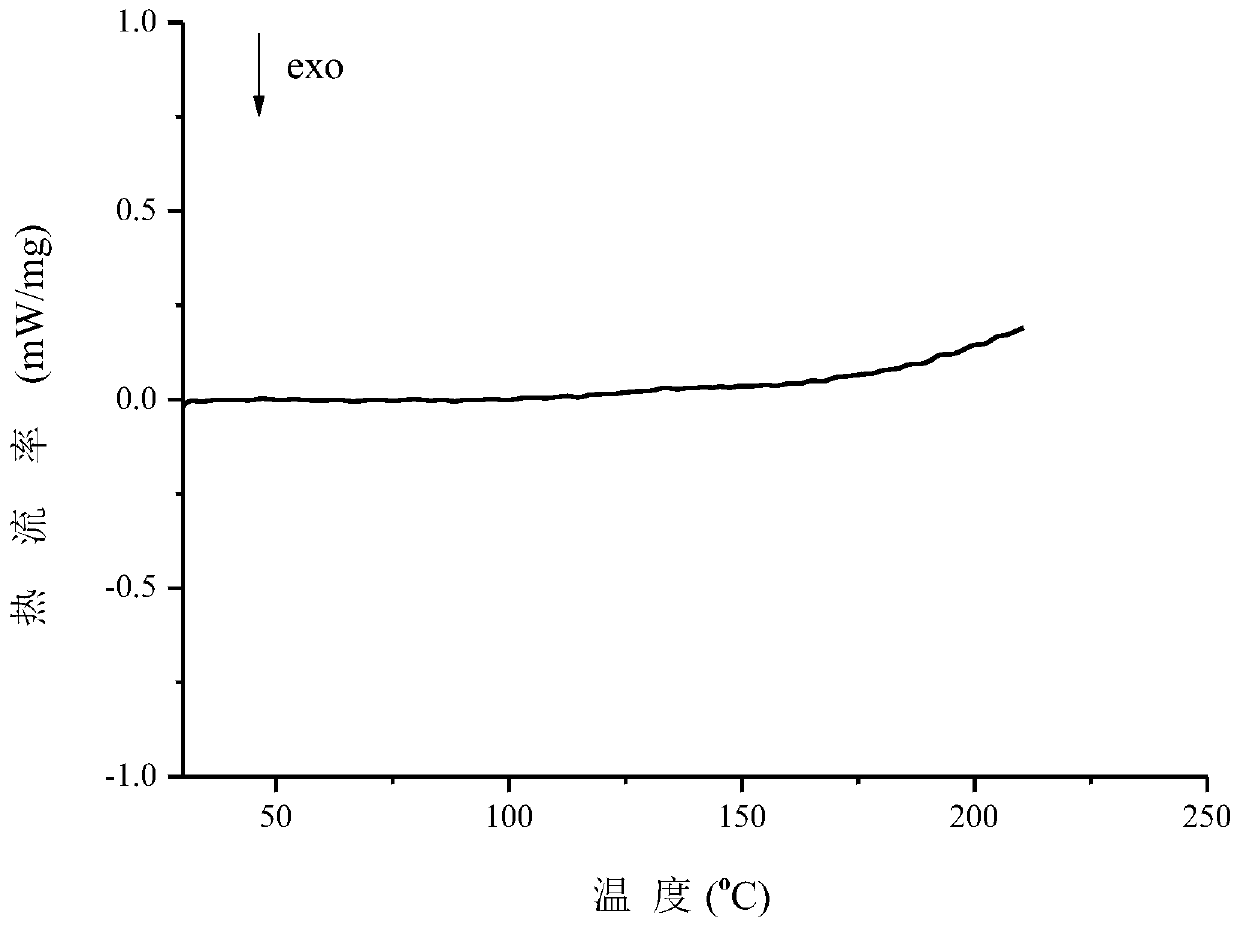
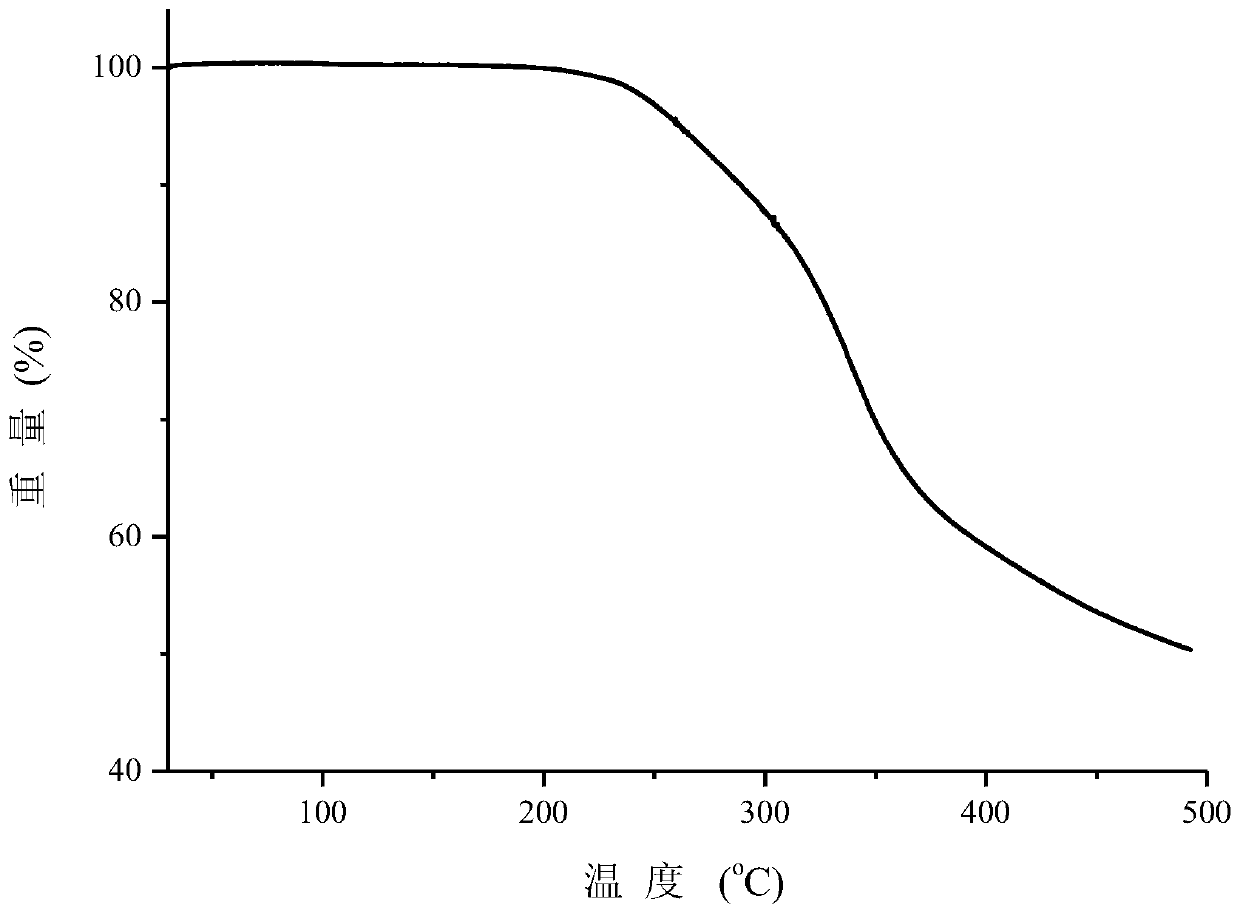
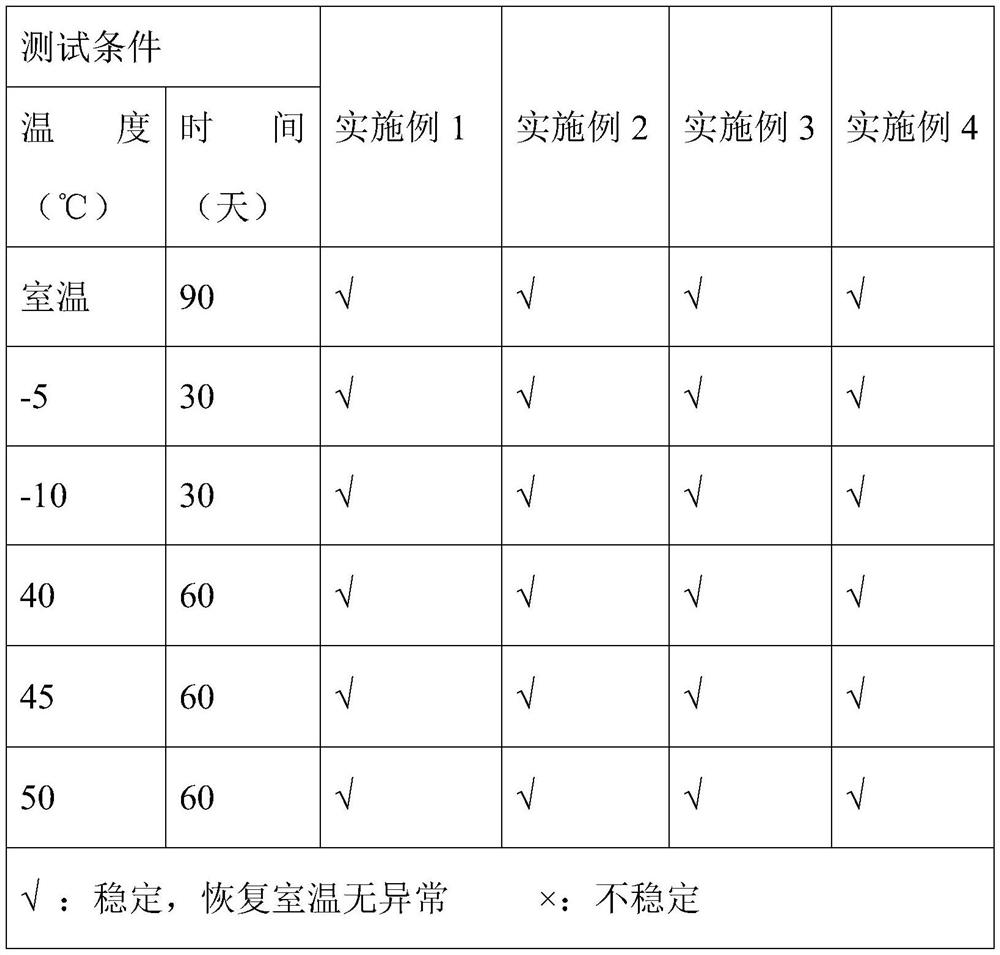
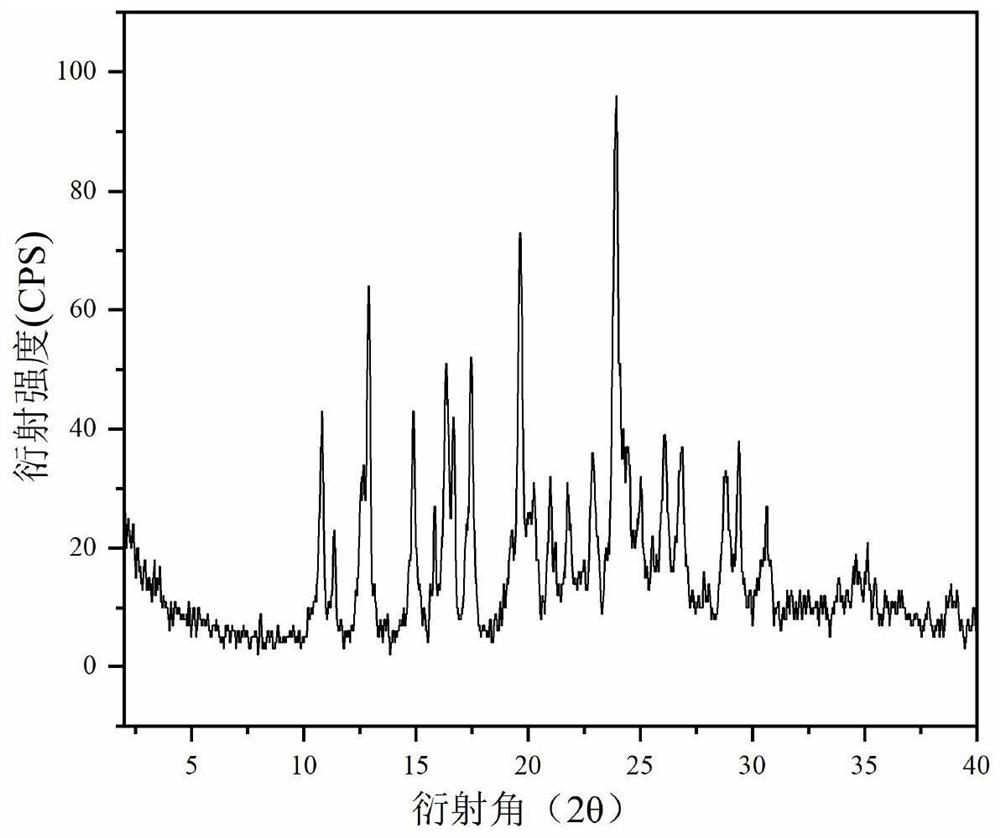
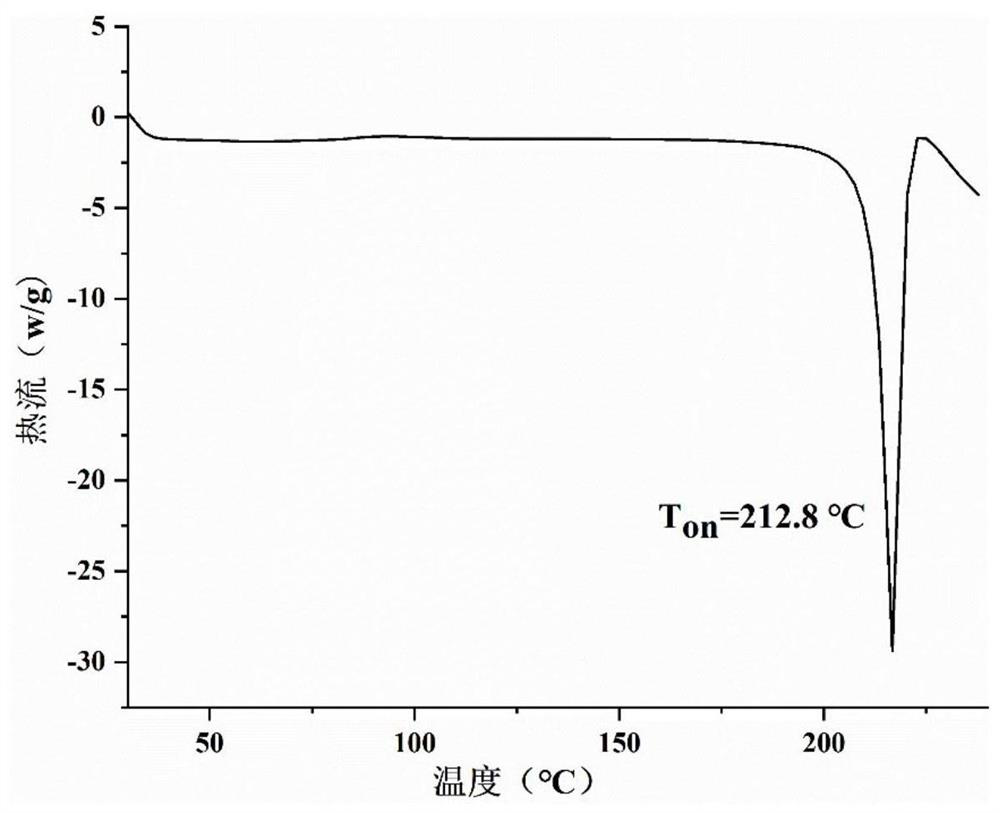
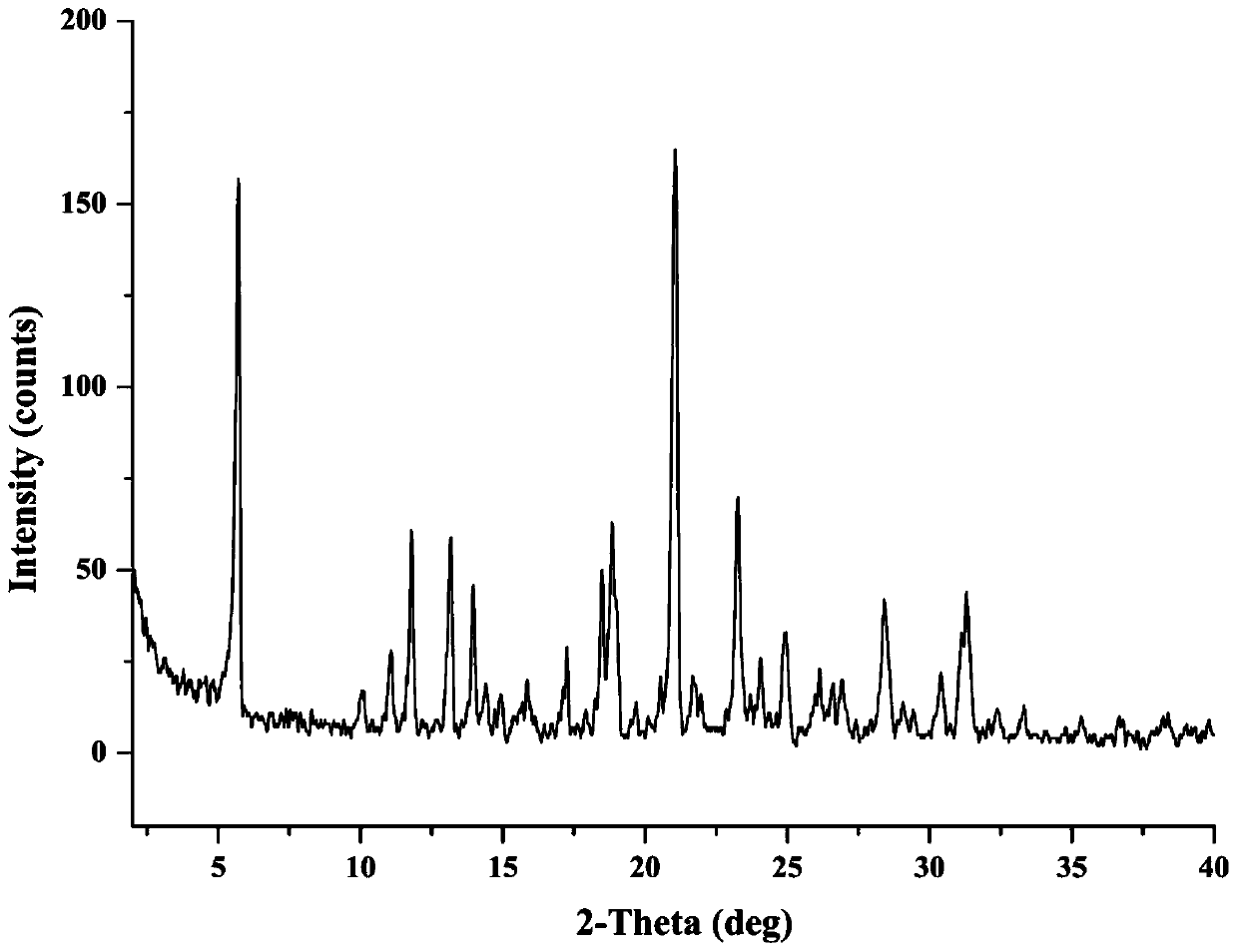
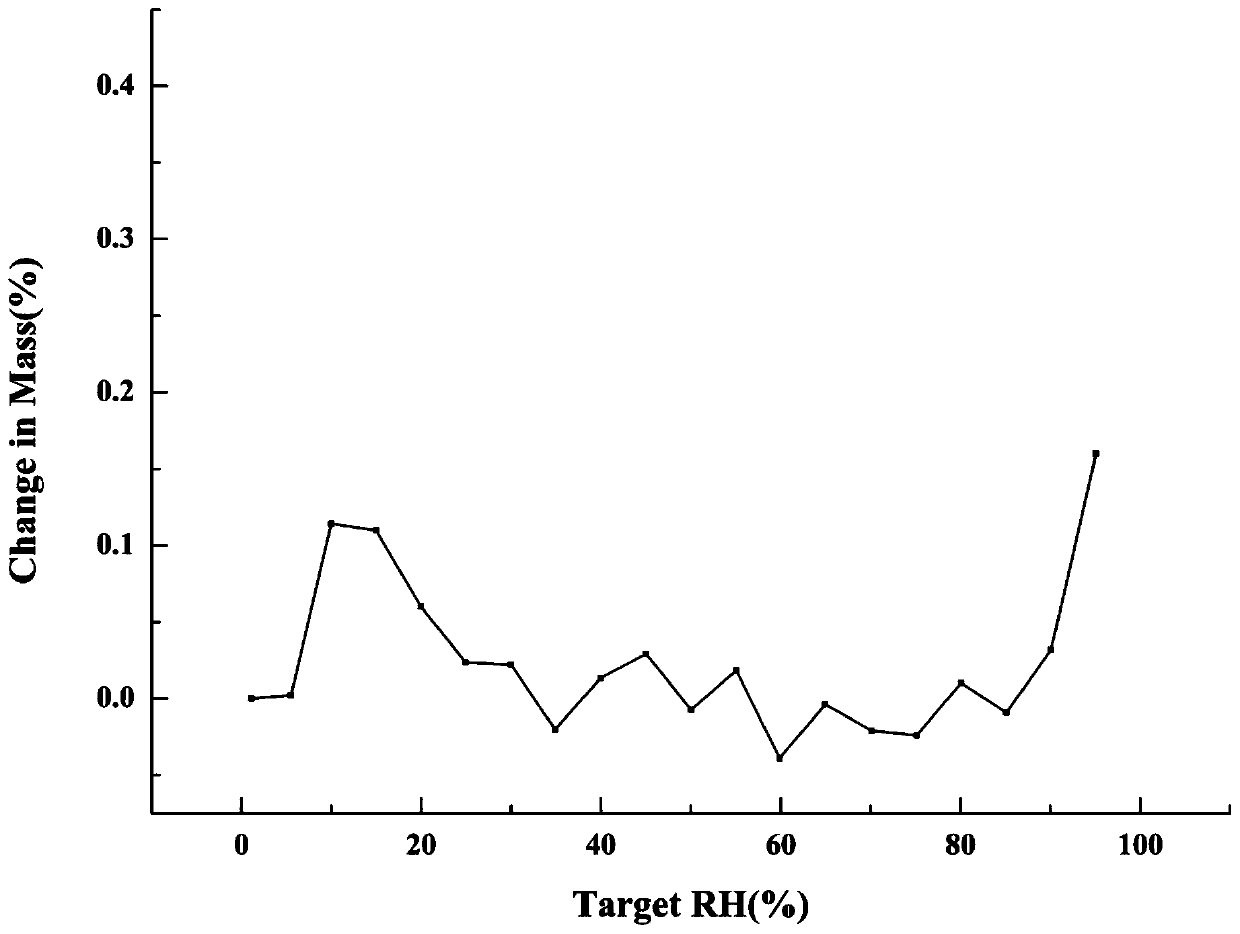
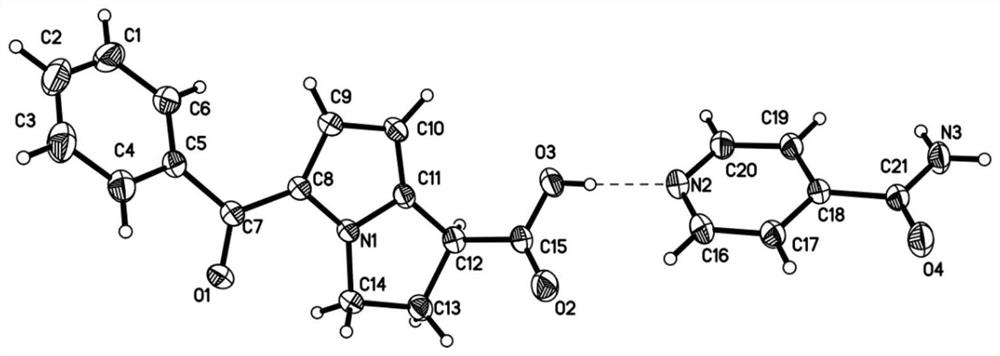
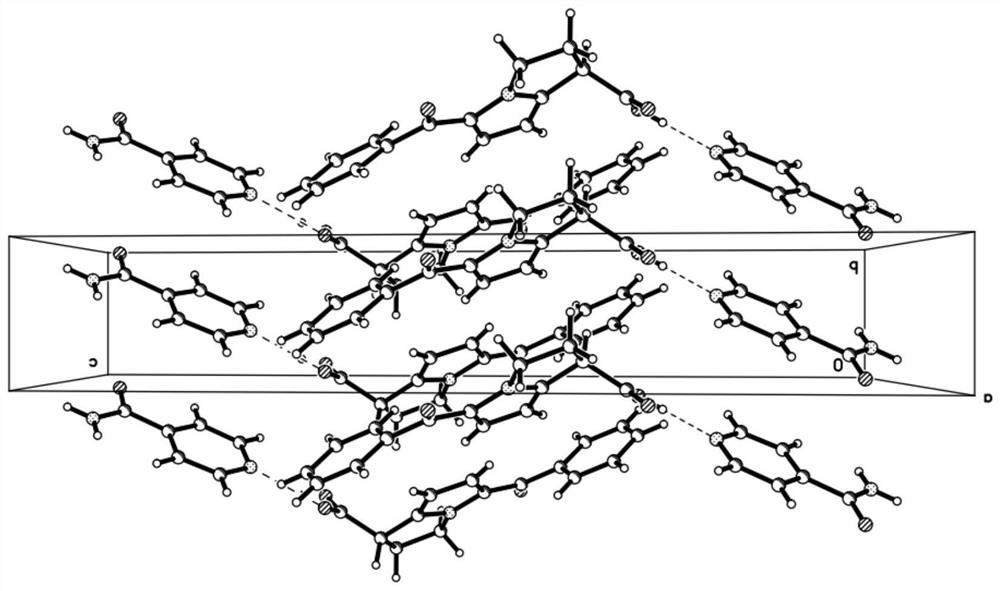
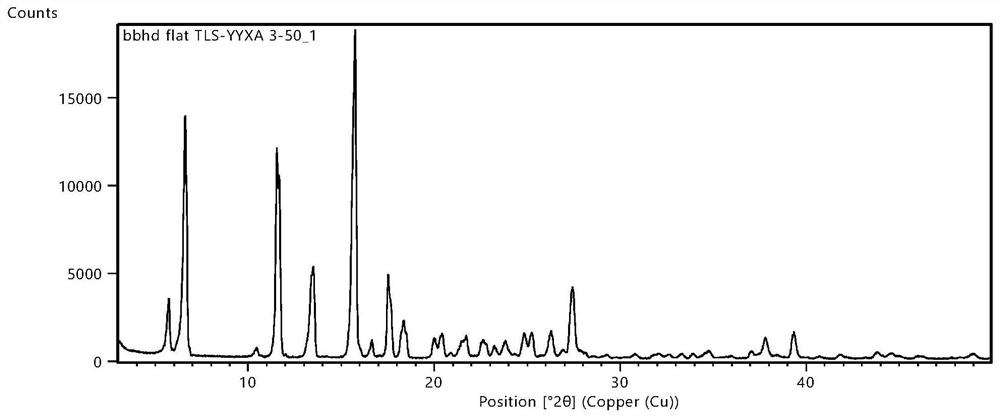
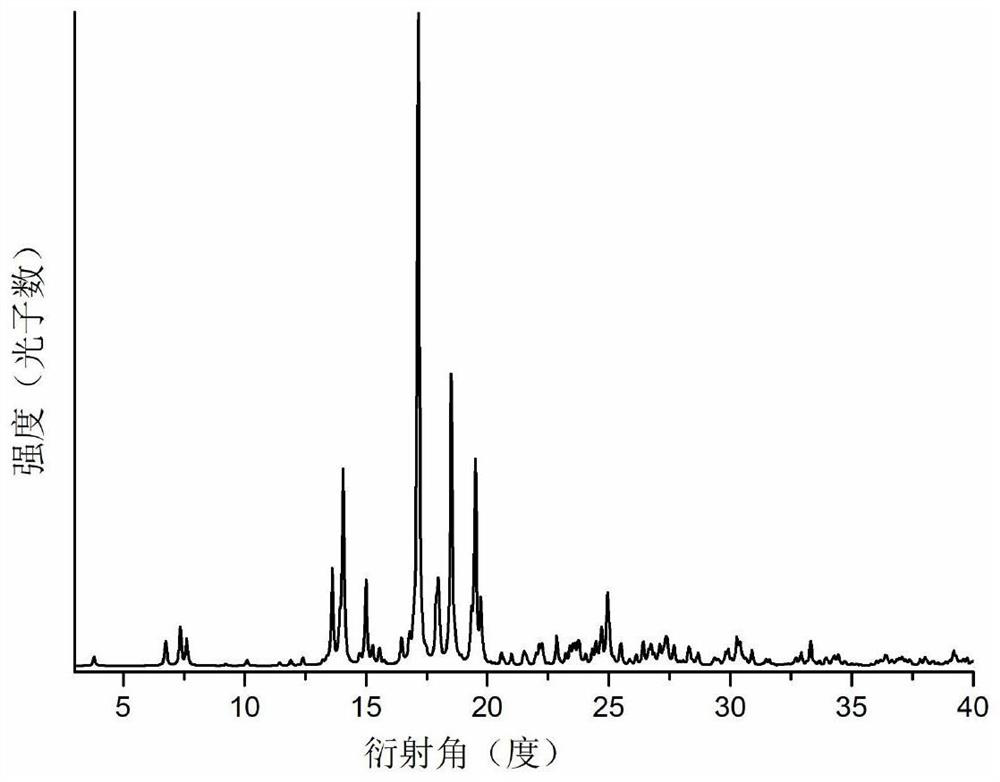
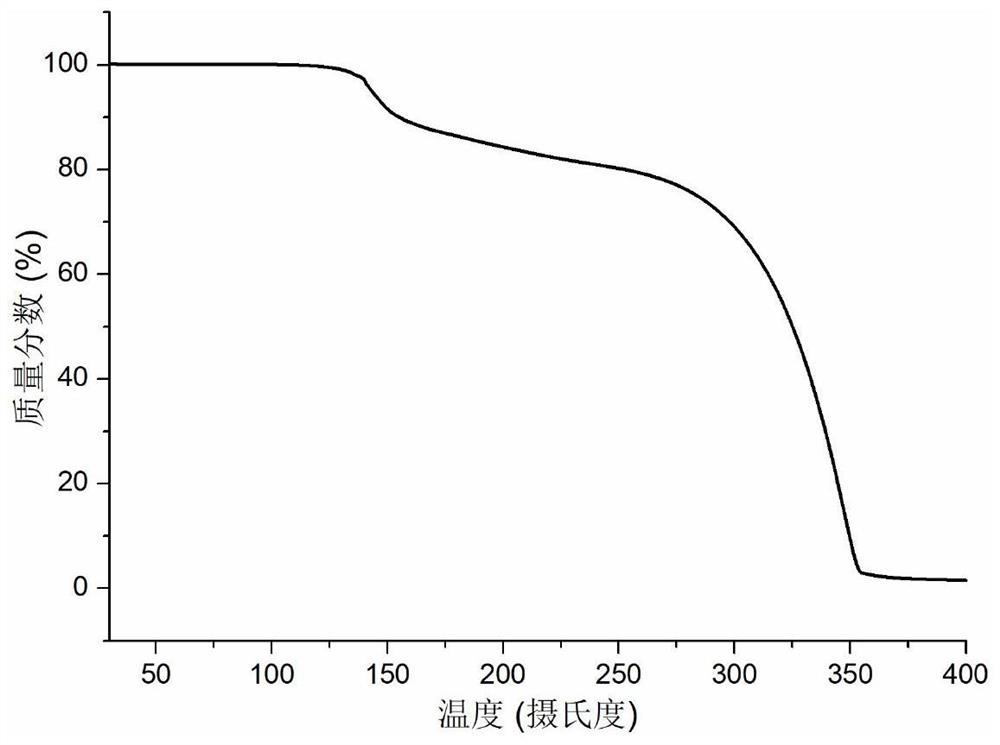
Patents
Literature
72results about How to "The crystallization process is easy to control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for improving mechanical properties of aramid fiber in supercritical fluid through stretching orientation
The invention relates to a method for improving mechanical properties of an aramid fiber in a supercritical fluid through stretching orientation. A supercritical carbon dioxide fluid is utilized for partially destroying interaction of a PPTA (poly-p-phenylene terephthamide) molecular chain in the aramid fiber under the action of certain tension, and the molecular chain is further oriented, so that the aramid fiber with good properties is obtained. The method for improving the mechanical properties of the aramid fiber in the supercritical fluid through stretching orientation mainly comprises the following steps: ensuring that the aramide fiber maintains certain tension in a closed container, introducing CO2 into the container at a certain temperature, so that the internal space of the closed container is in a supercritical CO2 state, carrying out swelling reaction for a period of time, and slowly releasing pressure, thus the highly stretching-oriented aramid fiber is obtained. The method for improving the mechanical properties of the aramid fiber in the supercritical fluid through stretching orientation guarantees that the aramid fiber is in a stretched state in a reaction process, so that orientation degree and crystallinity of a molecular chain are increased while stretching tension is changed, crystal particles are largened, and crystals are more and more complete.
Owner:DONGHUA UNIV +1
Method for producing large-sized zirconium ingot with the specification Phi of more than 600mm
The invention relates to a method for producing large-sized zirconium ingot with the specification Phi of more than 600mm, and the technical characteristics lie in that a consumable electrode with relatively uniform components is prepared by adopting dosing, mixing and vacuum plasma arc electrode assembly and welding technology, and vacuum consumable electric arc melting is carried out for at least twice by adopting technology of 'speed reducing melting' and 'component uniformity controlling' and the like under the conditions that the melting speed control parameter is 10-40kg / min and the arc stability control is carried out with the magnetic induction of 10-50 Gauss and the stable arc current alternate control time of 1-60 seconds. Compared with the prior art, the invention has the advantages that the large-sized zirconium ingot produced by the invention has uniform and stable chemical components and excellent ingot surface quality, is better than small ingot melted by vacuum consumable electric arc, and has the advantages of high yield, high production efficiency and being easy for producing large zirconium material and the like; and the invention is applicable to producing ingots for large zirconium material.
Owner:BAOJI TITANIUM IND CO LTD
Zirconium silicate powder and preparation method and application thereof
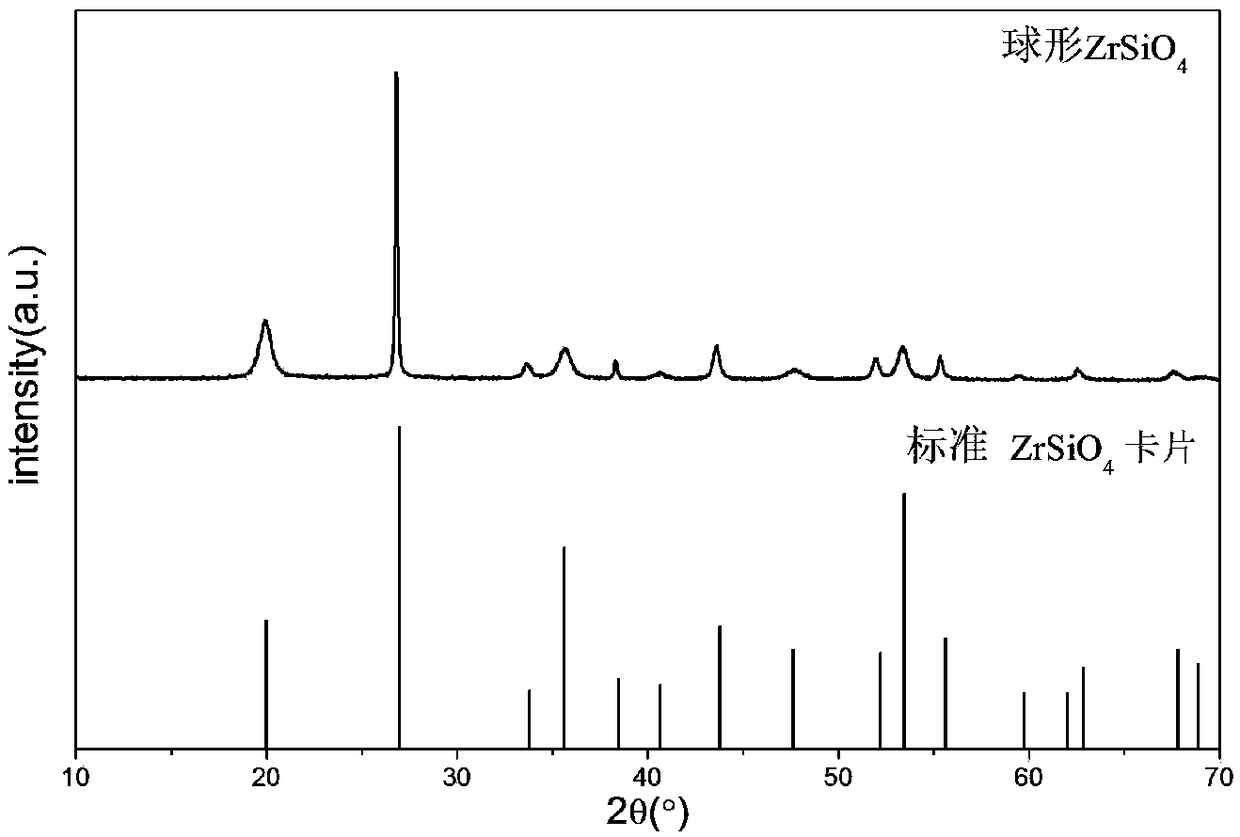
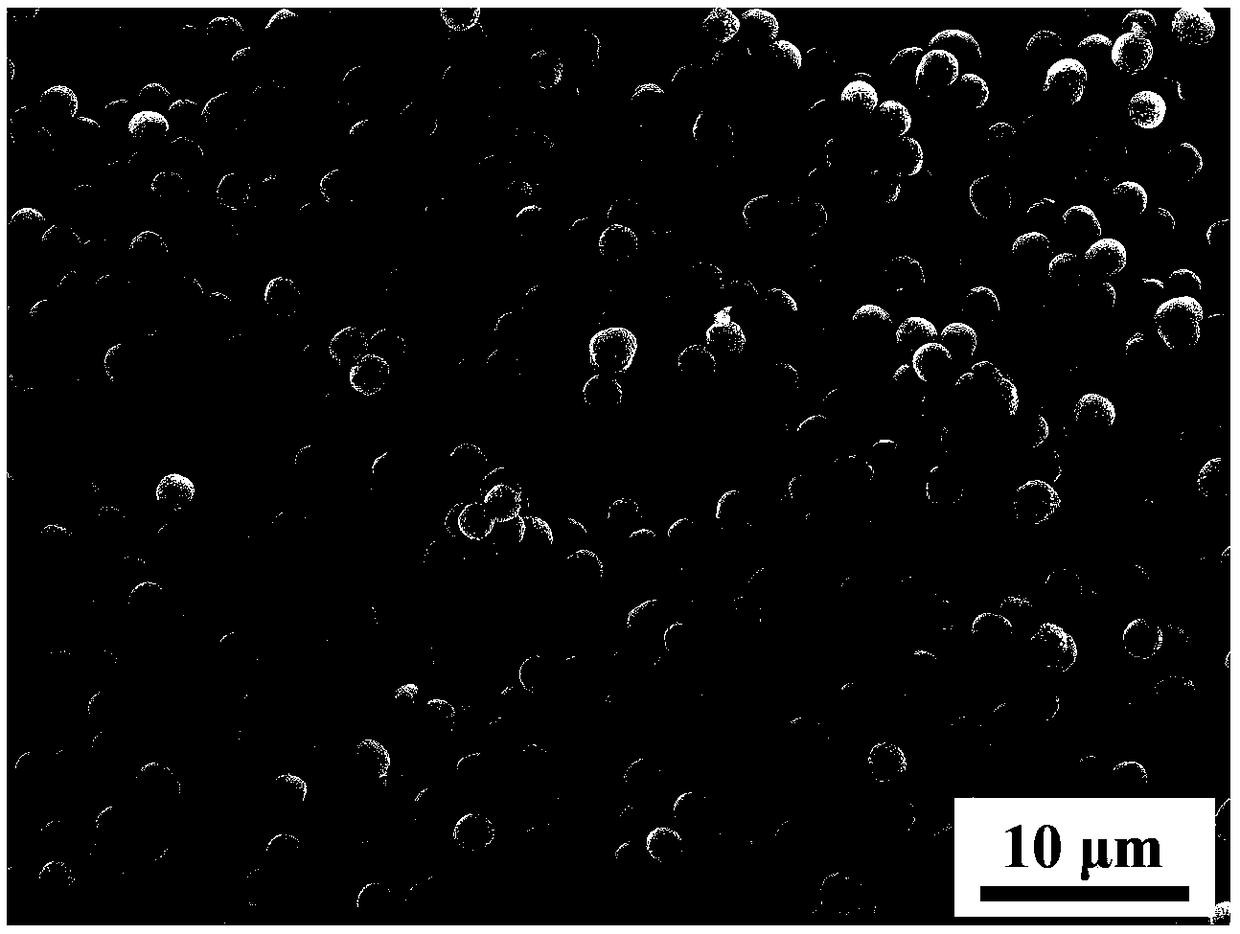
The invention provides a preparation method of zirconium silicate powder, and relates to the technical field of high-performance powder materials. The preparation method of the zirconium silicate powder comprises the following steps that 1, a zirconium source, acid and water are mixed to obtain a zirconium source solution; 2, a silicon source and a solvent are mixed to obtain a silicon source solution; 3, the zirconium source solution obtained in the step 1 and the silicon source solution obtained in the step 2 are subjected to mixing and prehydrolysis in sequence to obtain a precursor solution; 4, the precursor solution obtained in the step 3, a mineralizer and a surfactant are mixed for solvothermal reaction to obtain the zirconium silicate powder; the sequence of the step 1 and the step2 is not limited. The zirconium silicate powder prepared by adopting the method is 0.2-2.0-micron spheres.
Owner:HUBEI UNIV +1
Novel crystal form of cefuroxime sodium and preparation method of cefuroxime sodium crystal
ActiveCN104530084AAvoid degradationReduce decolorization filtration processOrganic chemistrySodium lactateCefuroxime Sodium
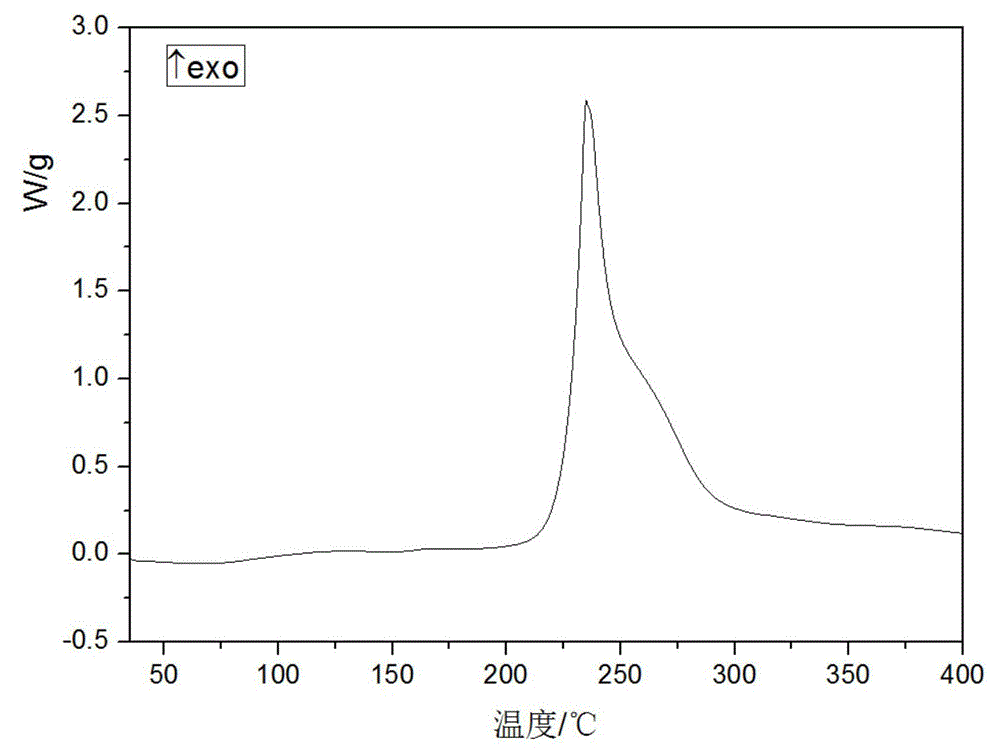
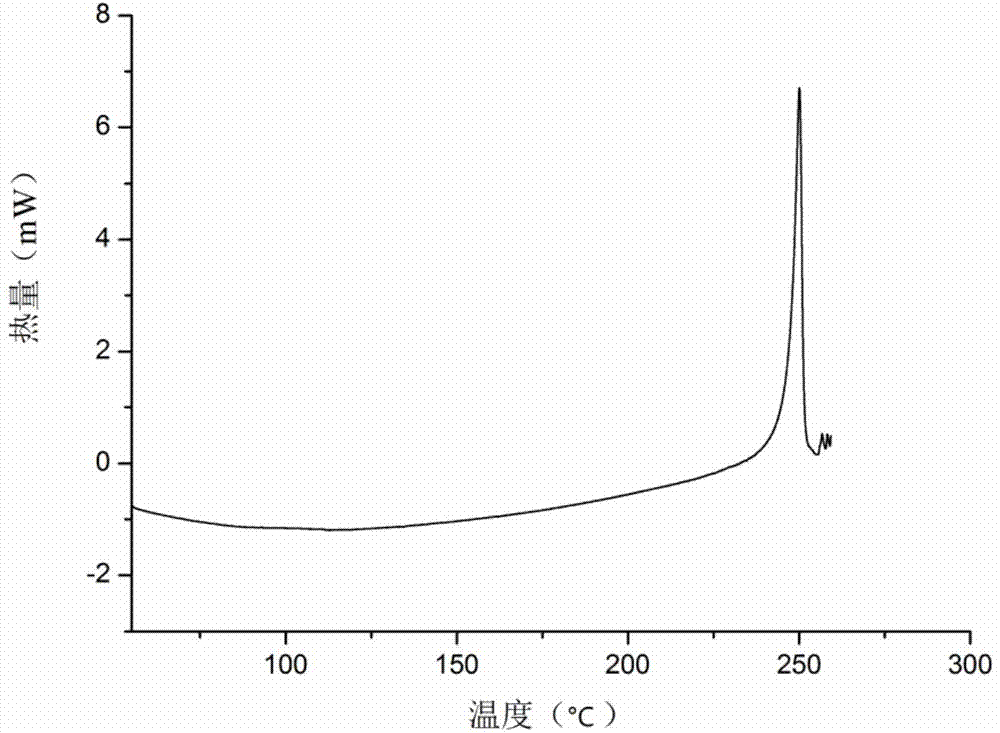
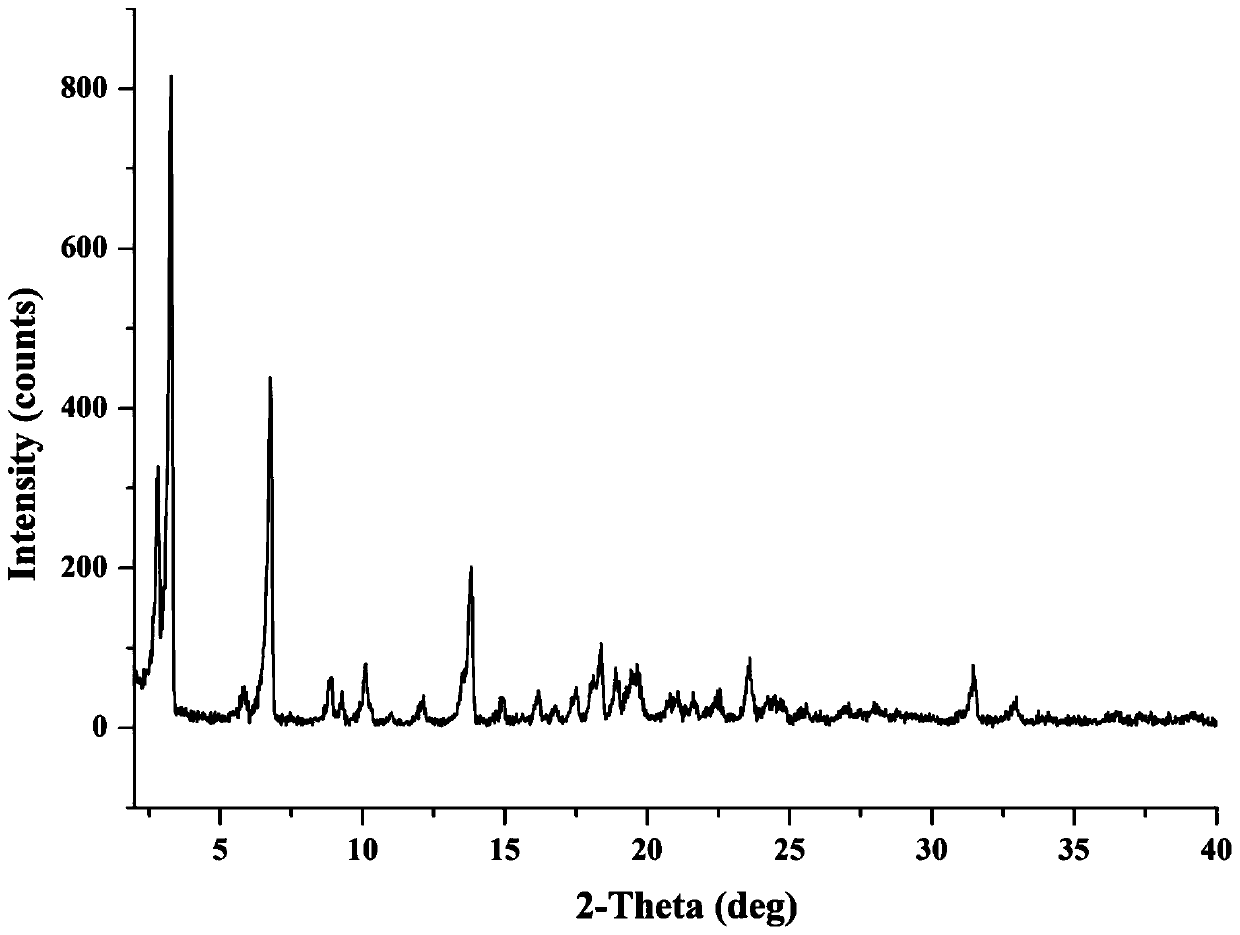
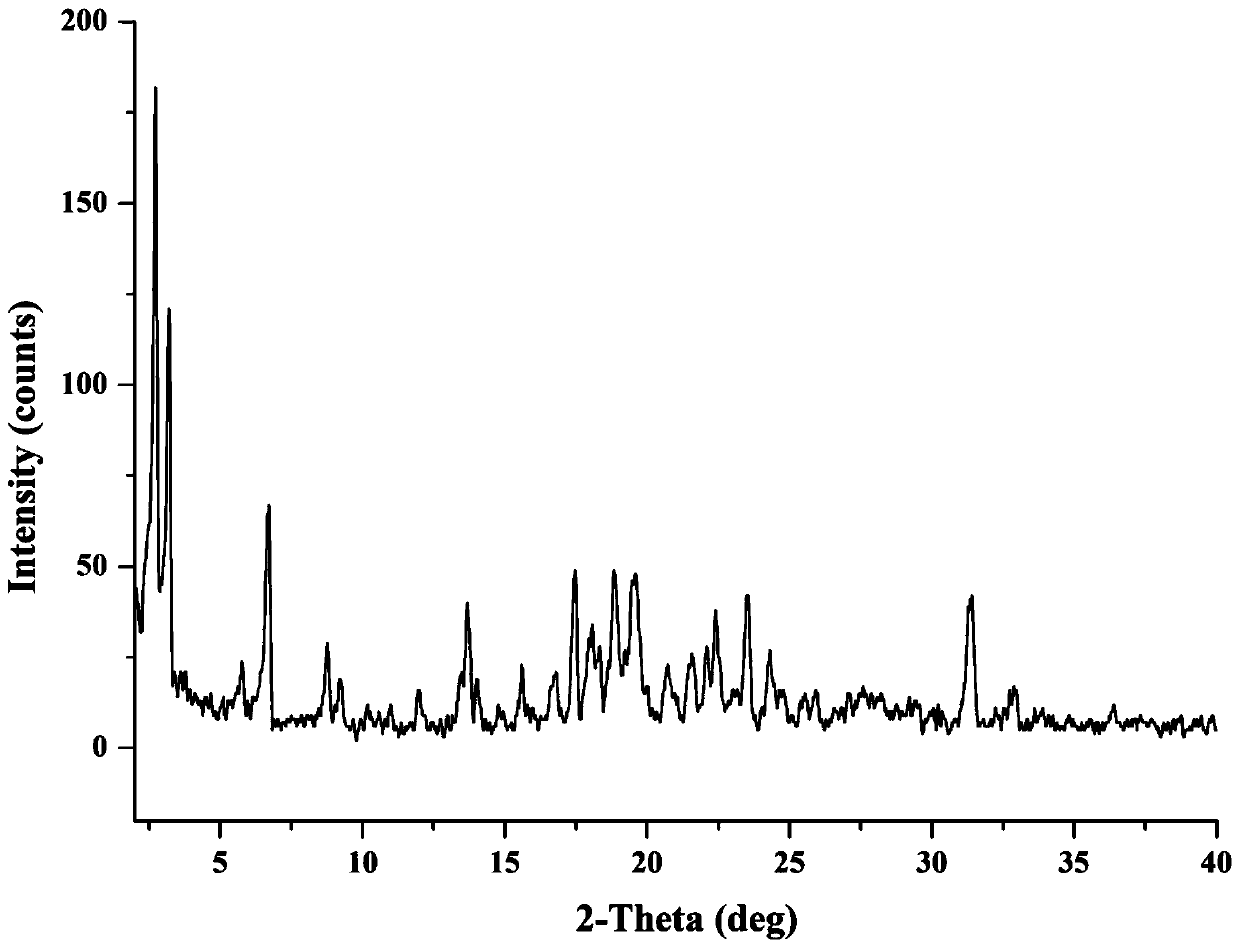
The invention provides a novel crystal form of cefuroxime sodium and a preparation method of cefuroxime sodium crystal. According to the novel crystal form crystal, the X-ray powder diffracting at the diffraction angles 2theta of 3.5+ / -0.2, 10.6+ / -0.2, 12.7+ / -0.2, 18.6+ / -0.2, 19.5+ / -0.2 and 22.4+ / -0.2 has characteristic peaks. The DSC graph has a characteristic peak at the angle of 235+ / -2 degrees. The method for preparing the novel cefuroxime sodium crystal comprises the following steps: adding 2.5-5g of cefuroxime acid with the purity of 99 percent into 100mL of a solvent for stirring at the temperature of 20-25 DEG C, drilling 0.2-0.4g / mL of sodium lactate-methanol solution for reacting until the pH value of the solution is 6.0-6.5; adding a solventing-out agent for performing solvent-out crystallization; and filtering, washing and drying the crystal mush, thereby obtaining the cefuroxime sodium crystallization product with the crystal form.
Owner:TIANJIN UNIV
Crystallizing method for obtaining I crystal form (+)-(S)-clopidogrel bisulfate
InactiveCN102050829AThe crystallization process is easy to controlHigh yieldOrganic chemistryAlcoholSolvent
The invention discloses a crystallizing method for obtaining I crystal form (+)-(S)-clopidogrel bisulfate. The method disclosed by the invention comprises the following steps: dissolving (+)-(S)-clopidogrel free alkali in an alcohol solvent, and dropwise adding an alcohol solution of concentrated sulfuric acid, thereby obtaining the I crystal form (+)-(S)-clopidogrel bisulfate by controlling the dropwise adding temperature, mixing time and recrystallization temperature.
Owner:北京华禧联合科技发展有限公司
Preparation of Ti-6Al-4V titanium alloy large-sized casting ingot
ActiveCN101476050AStable sequential solidification processSequential solidification process controllableMelting tankTi 6al 4v
The invention relates to a large-scale ingot casting method for preparing Ti-6Al-4V titanium alloy, the invention adopts multiple-time vacuum self-consuming smelting method on the basis of routine technique, adopts a 'constant speed' finished product smelting method and adopts smelting speed control parameters to control the technological parameters in the finished product smelting process.The invention is characterized in that the smelting speed control parameter is 20-32Kg / min; the real smelting weight is on-line measured each second or according to a set time and an instant smelting speed is the ratio of the real smelting weight and the measurement time interval; if the smelting speed is higher than a desired value, the smelting current is then reduced; if the smelting speed is lower than the desired value, the smelting current is then increased. By using the control method, temperature field can be controlled effectively, distribution coefficient can be optimized, stabilization of the from and depth of the melting bath can be assured, which make the ingot casting sequence and solidification process stable and controllable, and homogeneity of components of large-scale ingot casting can also be assured.
Owner:BAOJI TITANIUM IND CO LTD
Olaparib and malonic acid eutectic crystal and preparation method thereof
PendingCN111825621AImprove oral absorption efficiencyImprove apparent solubilityOrganic active ingredientsOrganic chemistry methodsMalonic acidPhysical chemistry
The invention discloses an olaparib and malonic acid eutectic crystal and a preparation method thereof. In the eutectic crystal, the molar ratio of olaparib to malonic acid is 1: 1; the eutectic X-raypowder diffraction pattern has characteristic peaks when the 2theta value is at the positions of 9.8 + / -0.2 degrees, 12.4 + / -0.2 degrees, 12.6 + / -0.2 degrees, 13.4 + / -0.2 degrees, 16.2 + / -0.2 degrees, 17.5 + / -0.2 degrees, 18.7 + / -0.2 degrees and 21.0 + / -0.2 degrees. The eutectic preparation method is simple in process, easy to control the crystallization process, good in reproducibility and suitable for industrial production. Compared with olaparib free alkali, the eutectic crystal has higher apparent solubility, and is beneficial to improving the oral absorption efficiency of olaparib.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Olaparib and maleic acid cocrystal and preparation method thereof
ActiveCN111689905AImprove oral absorption efficiencyImprove apparent solubilityOrganic active ingredientsOrganic chemistry methodsSolubilityCombinatorial chemistry
The invention discloses an olaparib and maleic acid cocrystal and a preparation method thereof. The molar ratio of olaparib to maleic acid in the cocrystal is 1:1, and an X-ray powder diffraction pattern of the cocrystal has characteristic peaks when the 2theta values are 5.1+ / -0.2 degrees, 9.8+ / -0.2 degrees, 13.7+ / -0.2 degrees, 16.0+ / -0.2 degrees, 17.7+ / -0.2 degrees and 20.0+ / -0.2 degrees. The cocrystal preparation method provided by the invention is simple in process, easy to control the crystallization process, good in reproducibility and suitable for industrial production. Compared with olaparib free alkali, the cocrystal has higher apparent solubility, and is beneficial to improving the oral absorption efficiency of olaparib.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Apremilast and nicotinamide co-crystal as well as preparation method and application thereof
InactiveCN107721902AStable co-crystallizationEasy to operateOrganic active ingredientsAntipyreticX-rayRepeatability
The invention relates to an apremilast and nicotinamide co-crystal and a preparation method thereof. The co-crystal is subjected to comprehensive characterization by applying means including X-ray powder diffraction analysis, thermogravimetric analysis, differential scanning amount thermal analysis and the like, and the comprehensive characterization finds out that compared with apremilast, the co-crystal has better physical and chemical and drug formation performance. The preparation method of the apremilast and nicotinamide co-crystal is simple, easy to control and good in repeatability; theapremilast and nicotinamide co-crystal can be stably obtained.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
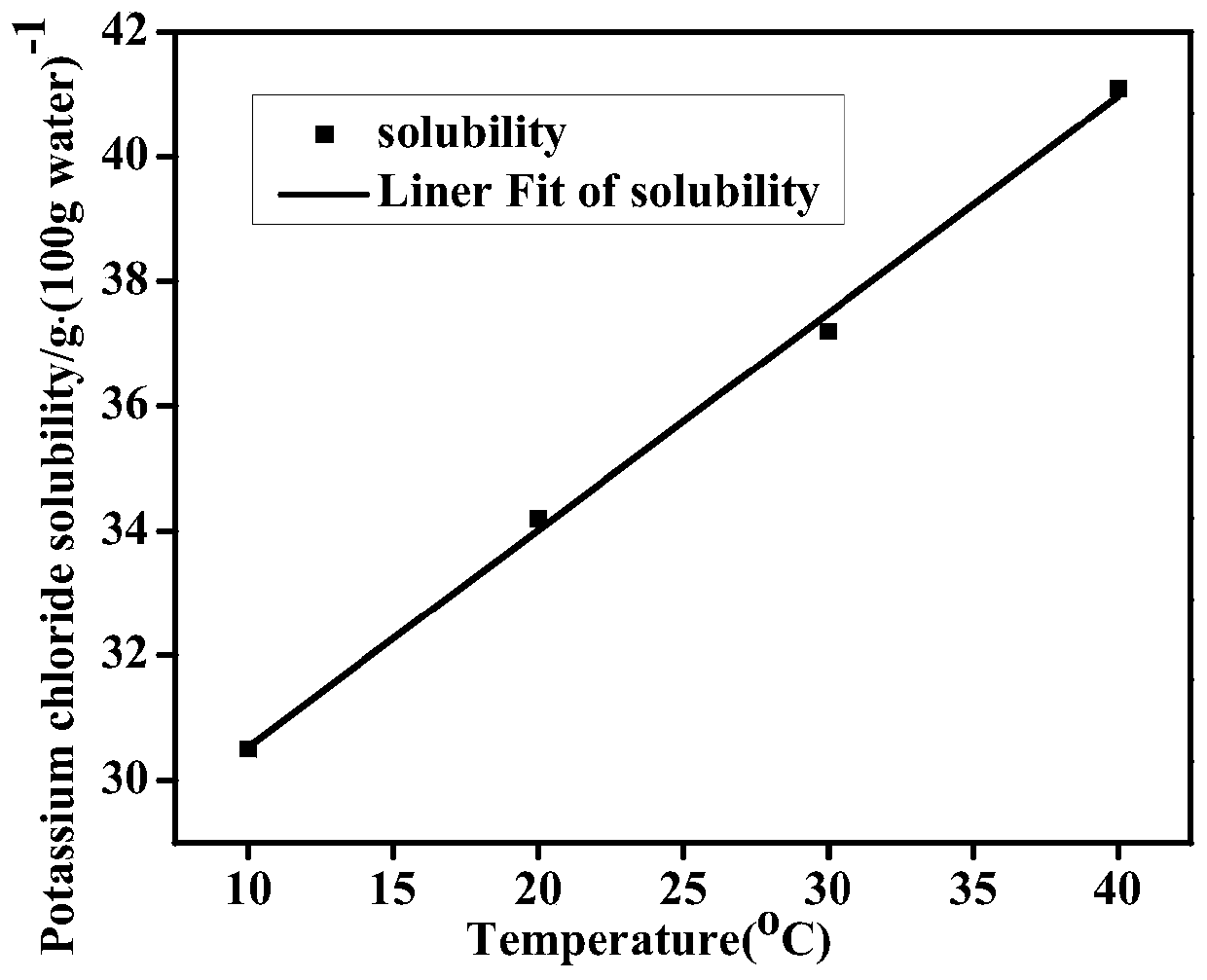
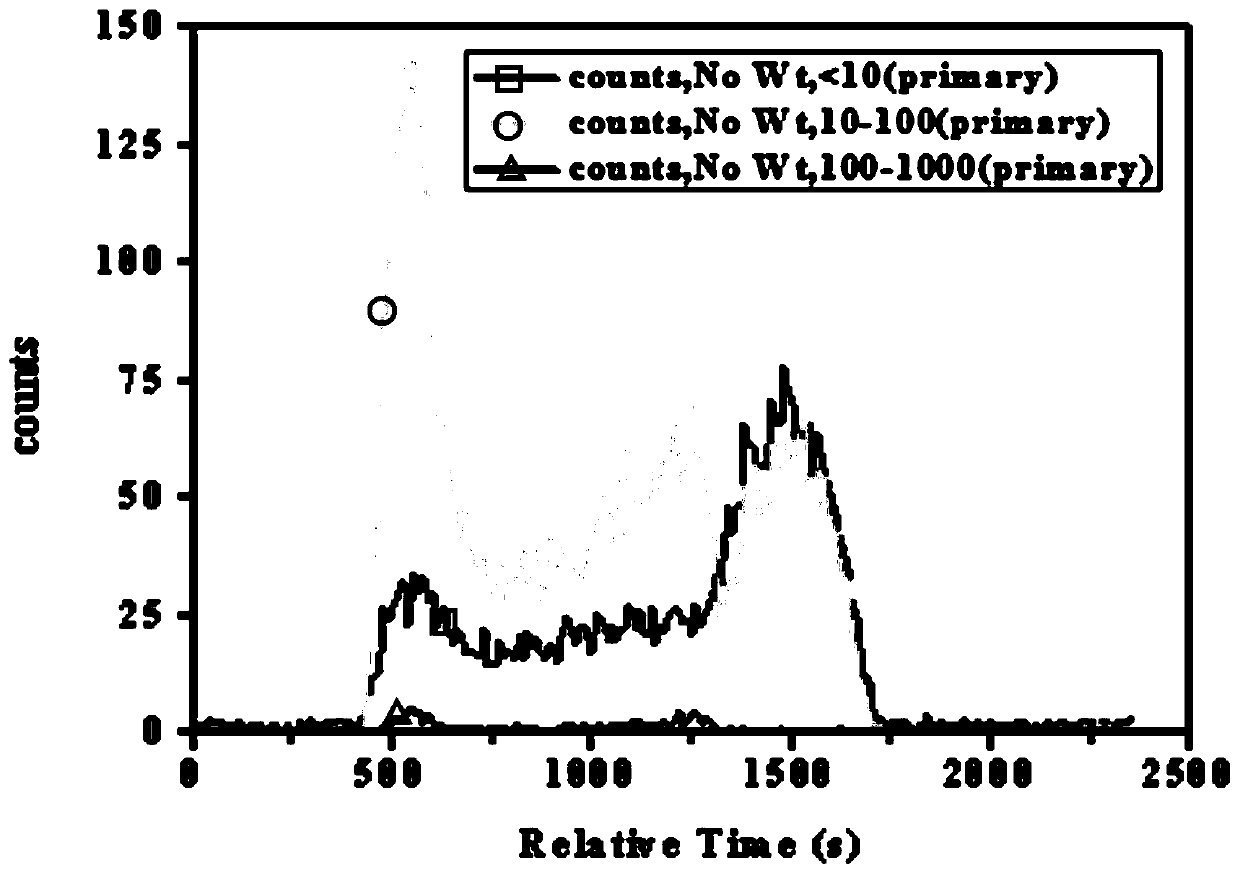
Method for optimizing crystallization of potassium chloride based on online monitoring system
InactiveCN110152343AHigh crystallinityImprove product qualitySolution crystallizationCrystallization by component evaporationIonChemistry
The invention discloses a method for optimizing the crystallization of potassium chloride based on an online monitoring system, and belongs to the field of potassium chloride production. A static technology is adopted to detect solubility data and supersolubility data of potassium chloride, the focused beam reflectance measurer (FBRM) and online particle size analyzer (PVM) monitoring system to detect the metastable zone of potassium chloride crystallization, the solubility of the potassium chloride gradually increases with the increase of the temperature, increase of the cooling rate widens the width of the metastable zone of potassium chloride, increase of the stirring speed narrows the width of the metastable zone of potassium chloride, and reduction of the addition amount of seed crystals narrows the width of the metastable zone of potassium chloride. The problem that existing optimization of the crystallization process of potassium chloride tends to focus on the influences of supersaturation, impurities and other ions on the crystal habits of potassium chloride crystal, but does not achieve a good crystallization optimization effect and spontaneous nucleation and secondary nucleation still exist is solved in the invention.
Owner:QINGHAI UNIV FOR NATITIES
Cooling unit for modification process of long glass fiber reinforced plastic
InactiveCN104827614AReduce usageThe crystallization process is easy to controlGlass fiberWater resources
A cooling unit for a modification process of long glass fiber reinforced plastic comprises a plurality of cooling water tanks arranged in parallel. Each cooling water tank is composed of a small tank and a large tank arranged below the small tank; the temperature of water in the large and small tanks decreases gradually along a moving direction of strips; grilles are disposed at two ends of the top of the small tank and are composed of a plurality of circular columns; a gap between every circular columns is used as a passage allowing passage of the strips; circulating pumps are disposed in the large and small tanks and provide water to the small tank through a pipe, the water in the small tank can flow back into the large tank through the grilles, and the water level of the small tank is lower than the horizontal plane which the strips pass by. The cooling unit has the advantages that the multiple cooling tanks of different temperatures are used to gradually cool the strips, defective rate of the strips can be evidently decreased, lean production is achieved, and waste of cooling water is avoided.
Owner:NANJING JINGJINYUAN TECHN IND
Olaparib-urea eutectic crystal and preparation method thereof
ActiveCN111995582AImprove oral absorption efficiencyImprove apparent solubilityUrea derivatives preparationOrganic active ingredientsPhysical chemistryPowder diffraction
The invention discloses an olaparib-urea eutectic crystal and a preparation method thereof. In the eutectic crystal, a molar ratio of olaparib to urea is 1: 2; in an X-ray powder diffraction pattern of the eutectic crystal, characteristic peaks occur when the value of 2theta is 6.4 + / - 0.2 degrees, 14.3 + / - 0.2 degrees, 15.0 + / - 0.2 degrees, 18.6 + / - 0.2 degrees, 19.2 + / - 0.2 degrees, 24.3 + / -0.2degrees and 24.8 + / -0.2 degrees. The preparation method of the eutectic crystal provided by the invention is simple in process, a crystallization process is easy to control, good in reproducibility and suitable for industrial production. Compared with olaparib free alkali, the eutectic crystal has higher apparent solubility and is beneficial to improving the oral absorption efficiency of olaparib.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
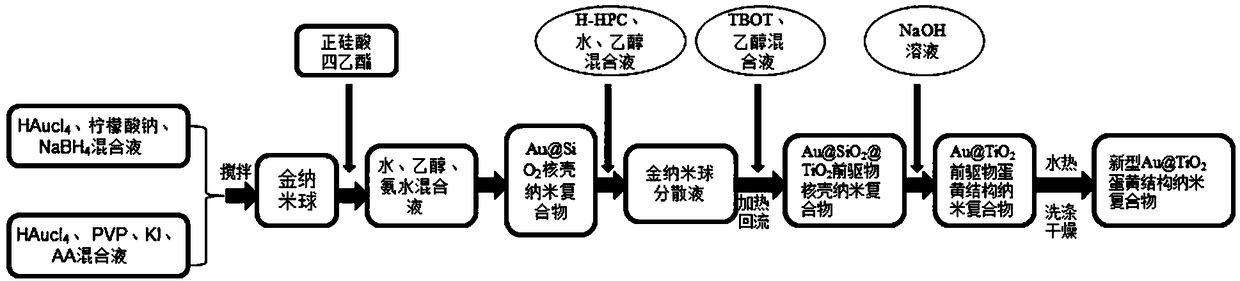
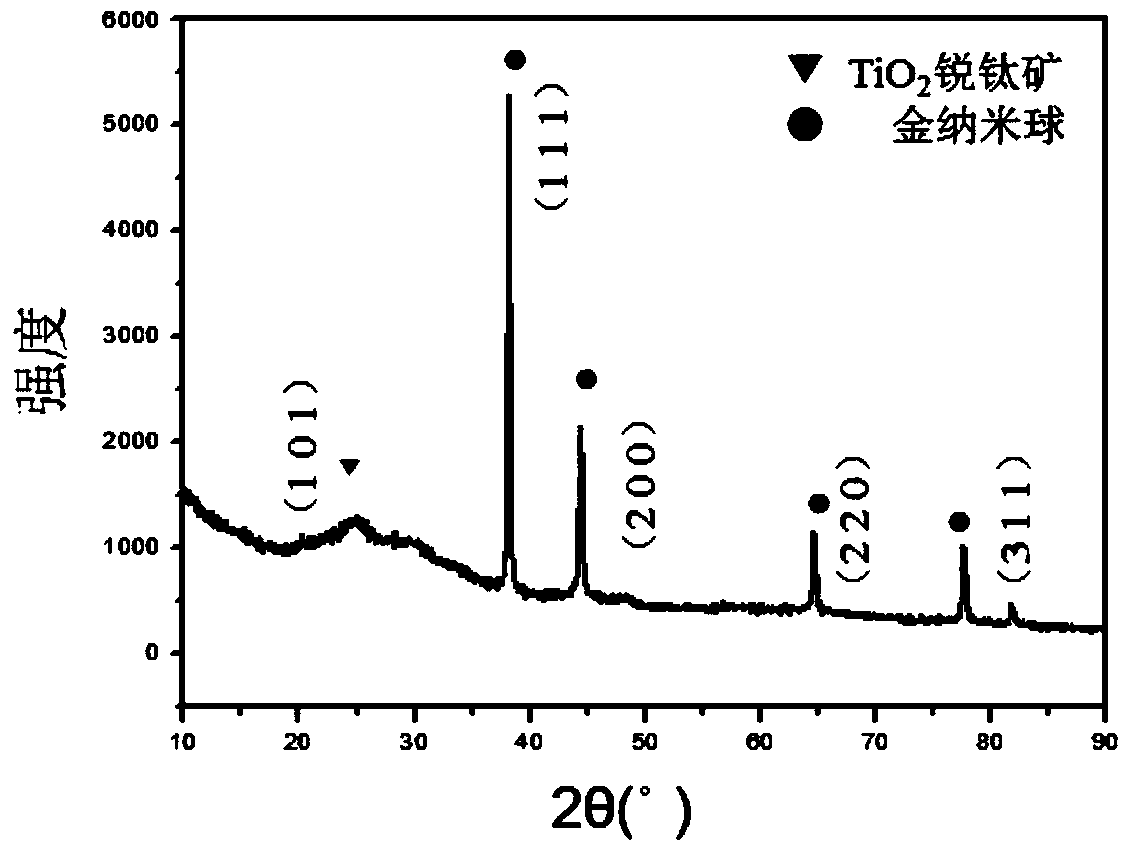
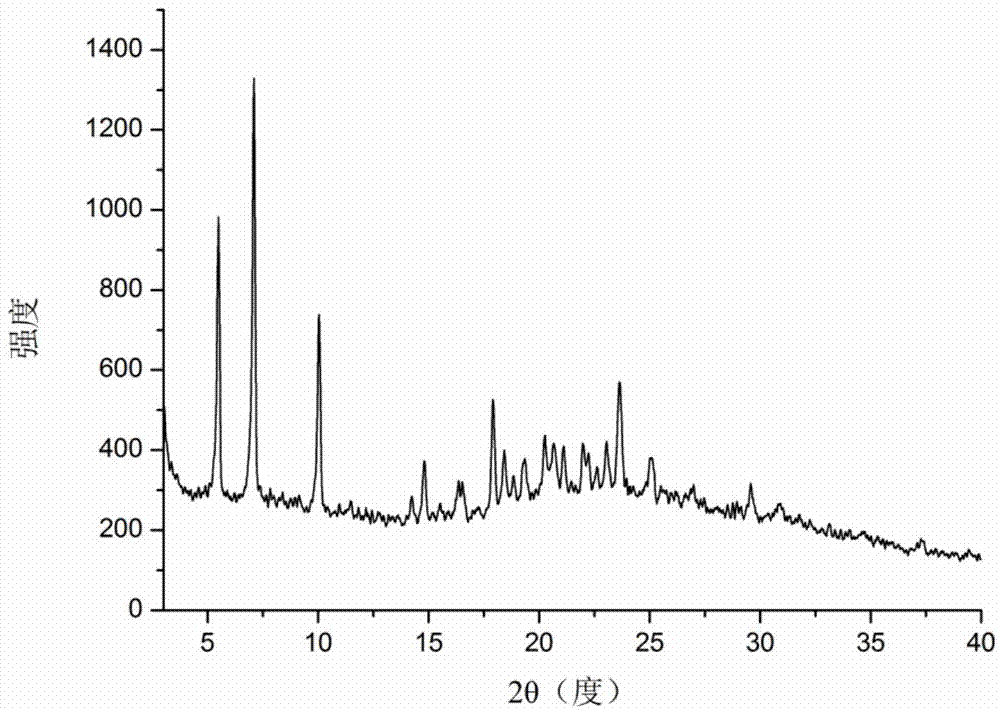
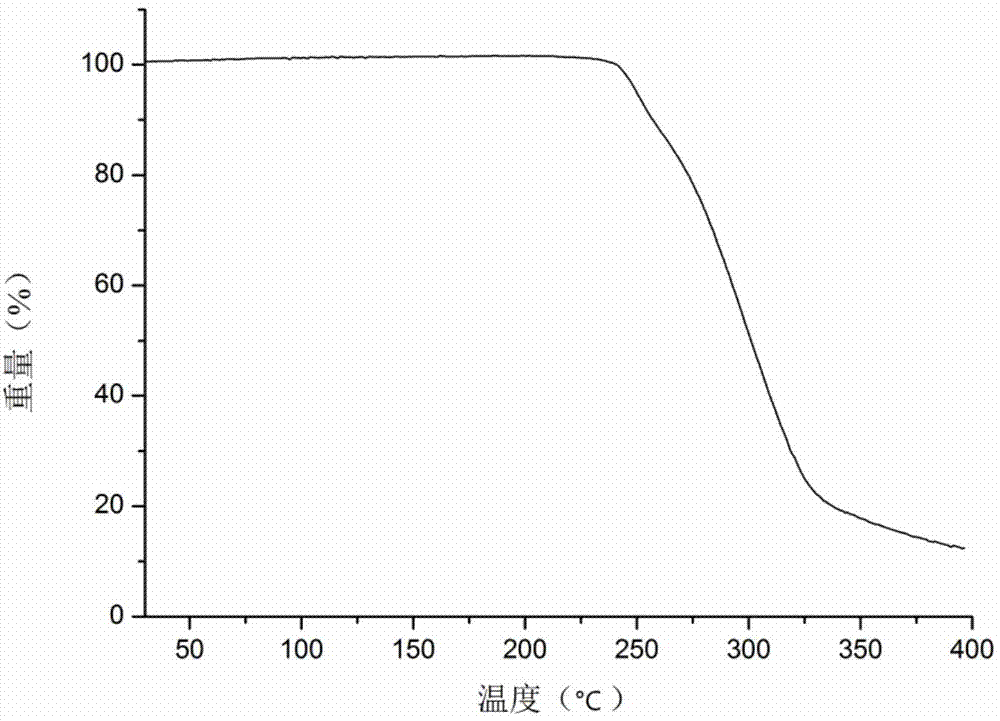
Au-TiO2 yolk-structured nanocomposite material and preparation method thereof
ActiveCN108906038AChanging the spectral absorption rangePlay a regulating roleWater/sewage treatment by irradiationWater contaminantsYolkMesoporous material
The invention relates to an Au-TiO2 yolk-structured nanocomposite material and a preparation method thereof. The preparation method comprises the following steps: firstly preparing a gold nanosphere;then coating the surface of the gold nanosphere with a silicon dioxide layer; coating the surface of the silicon dioxide layer with a titanium dioxide precursor mesoporous material layer; then removing the silicon dioxide layer; and finally carrying out hydro-thermal treatment to obtain the Au-TiO2 yolk-structured nanocomposite material with a sheet-like branch structure on the surface. The preparation method of the invention is simple in process, easy to operate, low in production cost, small in process pollution and suitable for large-scale production; and the prepared Au-TiO2 yolk-structured nanocomposite material has a unique movable core and has the sheet-like branch structure on the surface, which can increase the specific surface area of the material, greatly enhance the photocatalytic performance of the material and realize high utilization rate of solar energy.
Owner:NORTHEASTERN UNIV
Icariin form-alpha crystal, preparation method thereof and medicinal combination and application thereof
ActiveCN104844668AThe crystallization process is easy to controlHigh crystallinityOrganic active ingredientsNervous disorderX-rayCrystal
The invention belongs to the technical field of medicinal chemistry and particularly relates to an icariin form-alpha crystal, a preparation method thereof and a medicinal combination and application thereof. The crystal is represented through an X-ray powder diffraction diagram; the preparation method thereof comprises the step of heating icariin to 180 DEG C; the medicinal combination comprises the icariin form-alpha crystal and pharmaceutically acceptable carriers; the icariin form-alpha crystal is applied to the medicines for preventing and treating immune system diseases, tumor tissue diseases, genital system diseases, endocrine system diseases, bone tissue diseases, cardiovascular system diseases and nervous system diseases. The icariin form-alpha crystal provided by the invention has the advantages that the preparation method thereof is simple to operate, the crystallization process is easy to control, the crystallization degree is high, the reproducibility of the crystal is good and the stability is good.
Owner:ZHUCHENG HAOTIAN PHARMA
Metformin-pioglitazone salt, and preparation method and application thereof
InactiveCN110357871AEasy to operateThe crystallization process is easy to controlOrganic active ingredientsOrganic chemistrySolubilitySolvent
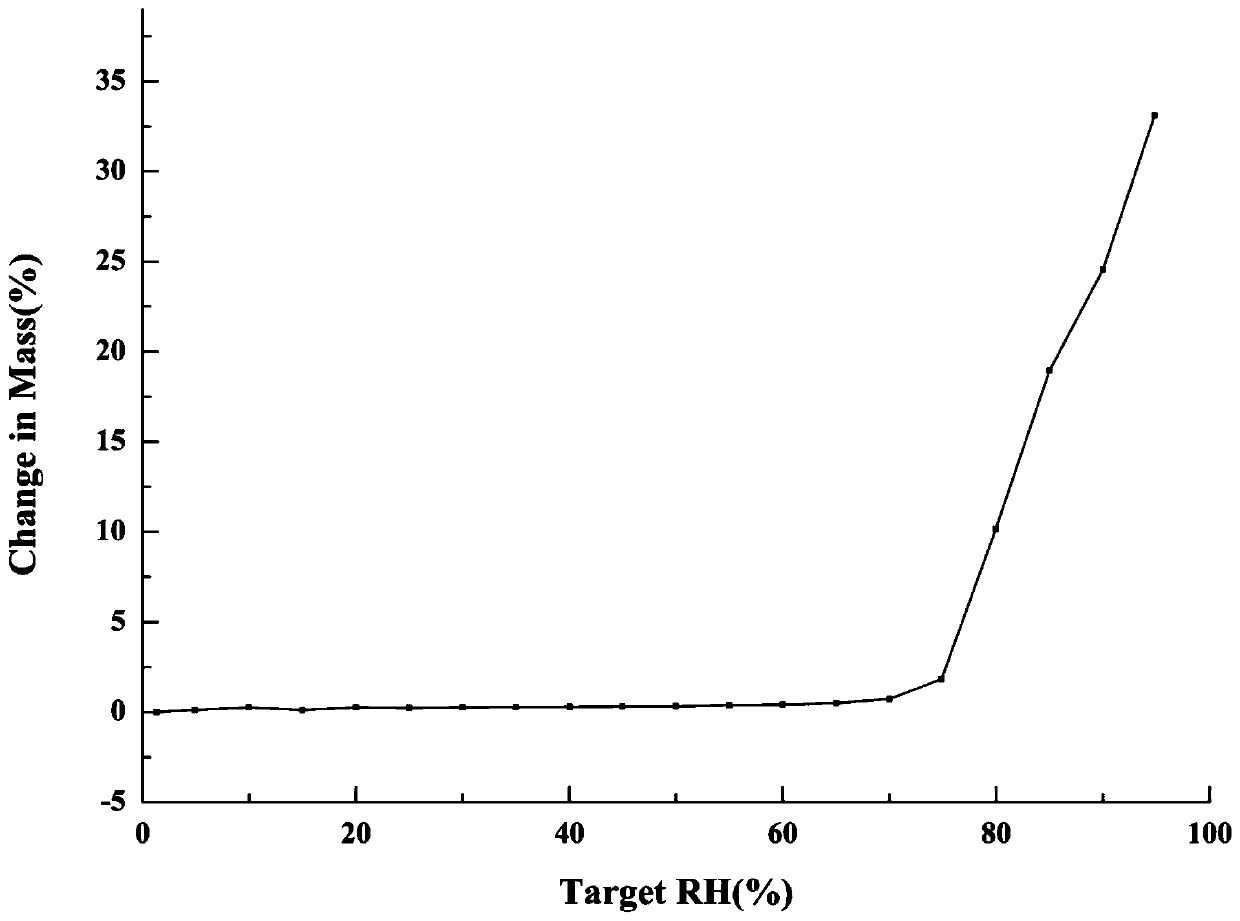
The invention relates to a metformin-pioglitazone salt, and a preparation method and an application thereof. The preparation method comprises the following steps: dissolving pioglitazone and metforminraw materials in a single solvent or a mixed solvent according to a molar ratio of 1:0.8 to 1:1.2; reacting and crystallizing the obtained mixture at 15-60 DEG C for 12-48 h; carrying out solid-liquid phase separation on the obtained product, and performing drying at 25-60 DEG C to obtain a solid sample of the metformin-pioglitazone salt. The metformin-pioglitazone salt provided by the inventionhas a greatly higher hygroscopic stability than the metformin product: the hydroscopic weight gain of the salt at a relative humidity of 95% is 33%, and the hydroscopic weight gain of the metformin single product under the same condition is 86%; the solubility of the metformin-pioglitazone salt in pure water is 1824 [mu]g / ml, and the solubility of the pioglitazone single product in the pure wateris less than 1 [mu]g / ml; and the preparation method of the salt has the advantages of simplicity in operation, easiness in control of the crystallization process, and good reproducibility of the salt.
Owner:TIANJIN UNIV
Sulfonylurea compound and metformin salts, preparation method and application
InactiveCN110804017AEasy to operateGood reproducibilityMetabolism disorderOrganic compound preparationDiabetes mellitusFluid phase
The invention relates to sulfonylurea compound and metformin salts, a preparation method and application. The preparation method comprises the following steps: dissolving a sulfonylurea compound and metformin into a solvent according to a mole ratio of (1:0.9):(1:1.1) so as to obtain a mixture; performing reaction crystallization on the mixture under a condition of 25-50 DEG C for 12-48 hours; andperforming solid-liquid phase separation on the obtained product, and performing drying, so as to obtain a salt type solid product. The sulfonylurea compound is gliquidone or glibenclamide. The gliquidone-metformin salt and the glibenclamide-metformin salt provided by the invention are applied to medicines for preventing and treating diabetes. The wet absorption capacities of the salt type products provided by the invention are greatly increased when being compared with that of metformin, that is, the hygroscopy gain of the metformin is 35% when the relative humidity is 75%, and the hygroscopy gains of the salt type products are smaller than 1%; and the dissolubility of the salt type products is greatly improved when being compared with that of the sulfonylurea compound, that is, the dissolubility of the sulfonylurea compound in water is smaller than 0.03mg / ml, and the dissolubility after salt formation is 10.61mg / ml and 16.78mg / ml respectively.
Owner:TIANJIN UNIV
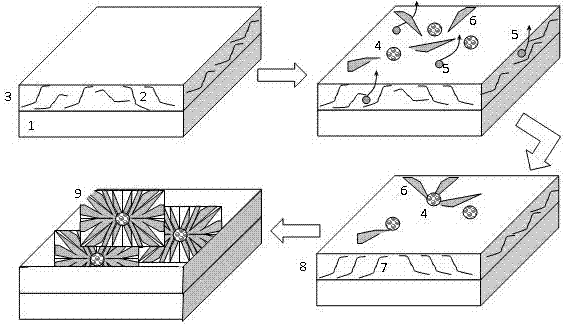
A method for regulating the growth of spherical rubrene crystal thin films by co-solvent of polymer-induced layer
ActiveCN106025101BUniform thicknessGood film formingSolid-state devicesSemiconductor/solid-state device manufacturingPolystyreneThin membrane
The present invention designs a method for regulating the growth of spherical rubrene crystals by the co-solvent of the polymer induction layer, that is, the method of regulating the orderly growth of rubrene molecules into spherical crystals through the induction layer of polystyrene (PS) under the co-solvent of chloroform . By spin-coating the polymer-induced layer (2) of chloroform solvent, a layer of substrate modification layer film (3) with uniform and directional arrangement is formed, and then the rubrene semiconductor layer (4) of chloroform solvent is drip-coated, using a co-solvent The microscopic arrangement of polymer molecules induces the growth of highly ordered arrangement of rubrene crystals, forming dense spherical rubrene crystals (9). The invention has simple operation and low cost, can obtain large-area, continuous and uniform rubrene spherical crystals, and lays a foundation for the growth of organic semiconductor thin film crystals with high mobility.
Owner:CHANGCHUN UNIV OF TECH
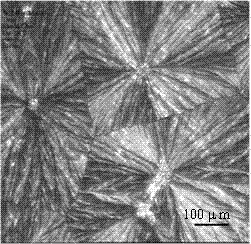
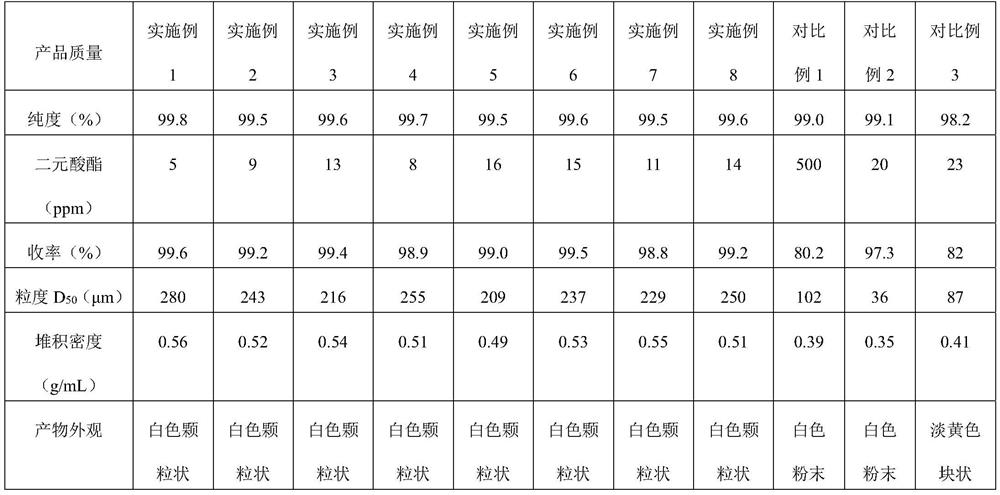
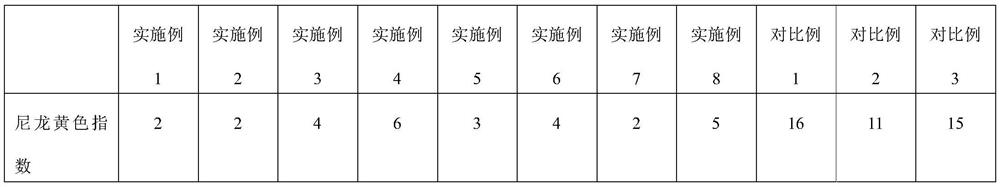
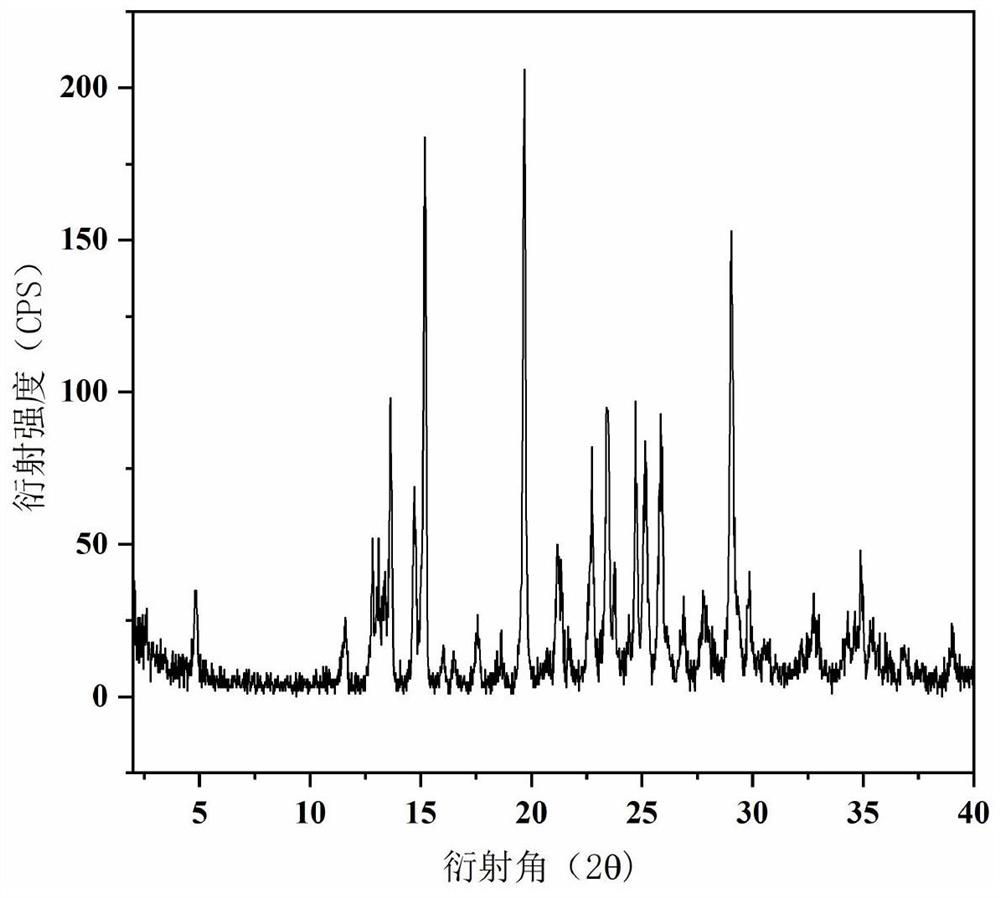
High-purity nylon 5X salt and purification method thereof
PendingCN111825557ALarge particlesHigh bulk densityAmino compound purification/separationCarboxylic compound separation/purificationDiamineAqueous solution
The invention relates to a high-purity nylon 5X salt and a preparation method thereof, wherein the purity of the nylon 5X salt reaches 99.5% or above, and the nylon 5X salt can be directly used for polymerization. According to the preparation method of the nylon 5X salt provided by the invention, pentamethylene diamine and nylon salt production processes are coupled together, and the nylon salt isproduced by a one-step method, so that the tedious and complex process flow is omitted, the equipment investment is reduced, and the production cost is saved; and the method provided by the inventionsolves the problem of low yield of nylon salt crystallization products by an aqueous solution method, the crystallization process is more effectively controlled, and explosion nucleation is avoided.
Owner:CATHAY R&D CENT CO LTD +1
Epalrestat-metformin salt hydrate as well as preparation method and application thereof
ActiveCN113277962AReduce humidityGood reproducibilityMetabolism disorderOrganic compound preparationSolubilityMedicine
The invention relates to an epalrestat-metformin salt hydrate crystal form and a preparation method thereof. The molecular formula of the hydrate is C19H26N6O4S2, and the molecular weight is 466.6. The crystallographic characteristics of the hydrate are as follows: the bond length a is 7.8711 (4), the bond length b is 8.3789 (4), the bond length c is 18.3489 (8), the bond angle alpha is 77.437 (4), the bond angle beta is 82.244 (4), the bond angle gamma is 66.896 (4), and V is 1084.7 (6). Compared with epalrestat, the epalrestat-metformin salt hydrate provided by the invention has the advantages that the dissolution rate and solubility are greatly improved, the problem of high hygroscopicity of metformin at the present stage is well solved, meanwhile, the preparation method is simple to operate, the crystallization process is easy to control, and the epalrestat-metformin salt hydrate is suitable for industrialization.
Owner:TIANJIN UNIV
Lenvatinib and p-hydroxybenzoic acid eutectic crystal and preparation method thereof
ActiveCN111233762AFast dissolution rateImprove apparent solubilityAntipyreticOrganic chemistry methodsLenvatinibPhysical chemistry
The invention discloses a lenvatinib and p-hydroxybenzoic acid eutectic crystal and a preparation method thereof. The eutectic crystal comprises lenvatinib and p-hydroxybenzoic acid in a molar ratio of 1: 1, an X-ray powder diffraction pattern of the eutectic crystal measured by Cu K alpha rays has characteristic peaks when diffraction angles 2theta are 6.3+ / -0.2 degrees, 10.6+ / -0.2 degrees, 12.4+ / -0.2 degrees, 14.6+ / -0.2 degrees, 17.5+ / -0.2 degrees and 18.4+ / -0.2 degrees. According to the preparation method, the lenvatinib is converted into a brand-new lenvatinib and p-hydroxybenzoic acid eutectic crystal for the first time, and the lenvatinib and p-hydroxybenzoic acid eutectic crystal has a relatively high dissolution rate and relatively high apparent solubility. The preparation method of the lenvatinib and p-hydroxybenzoic acid eutectic crystal is simple in process, easy to control the crystallization process, good in reproducibility, suitable for industrial production and wide in application prospect.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
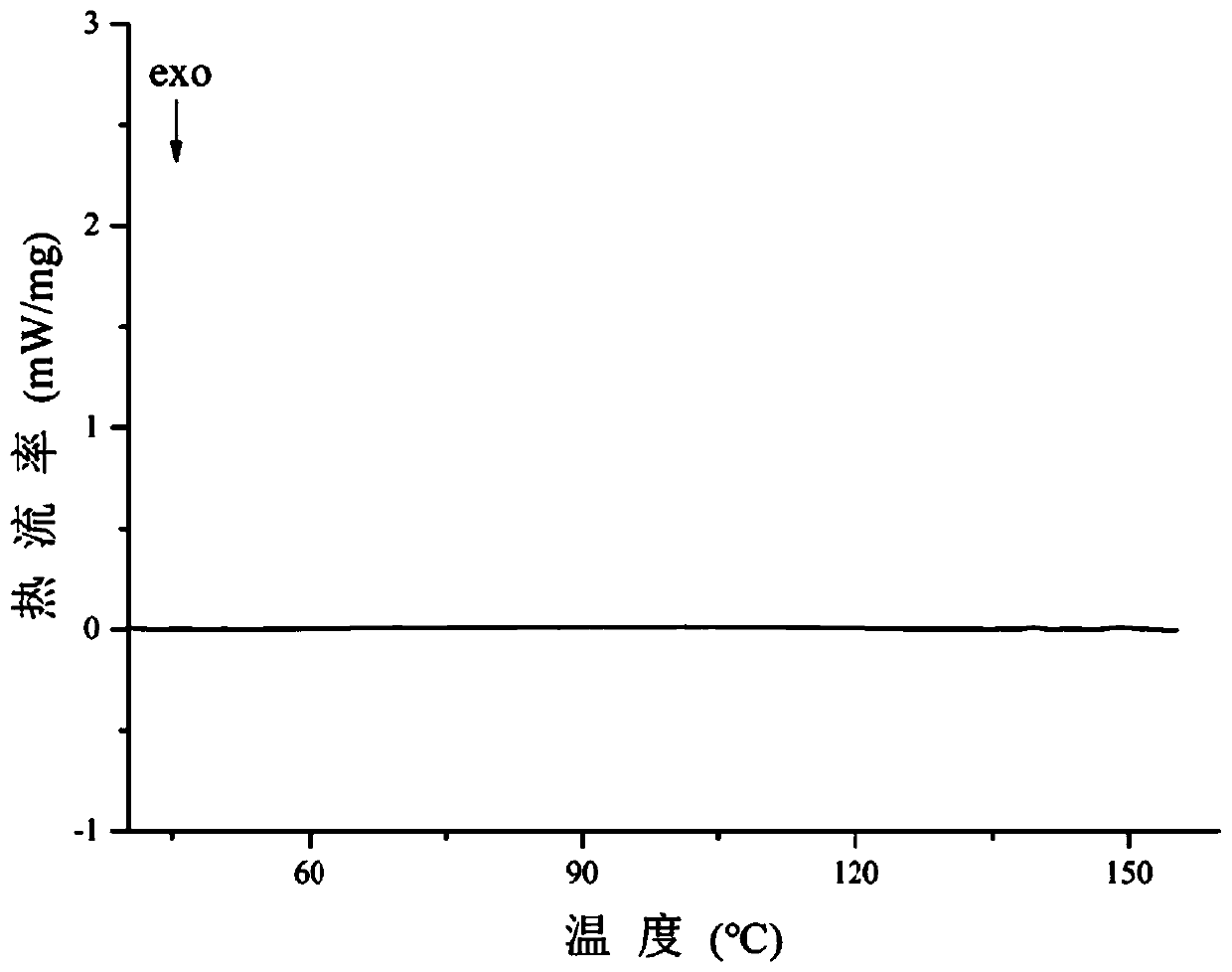
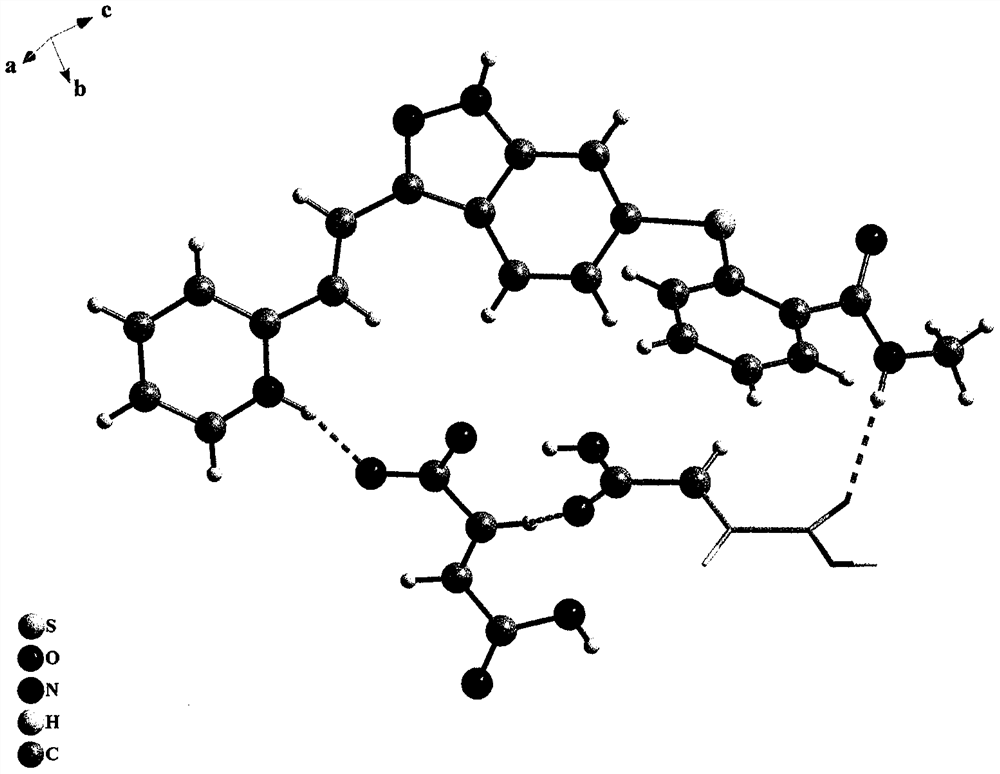
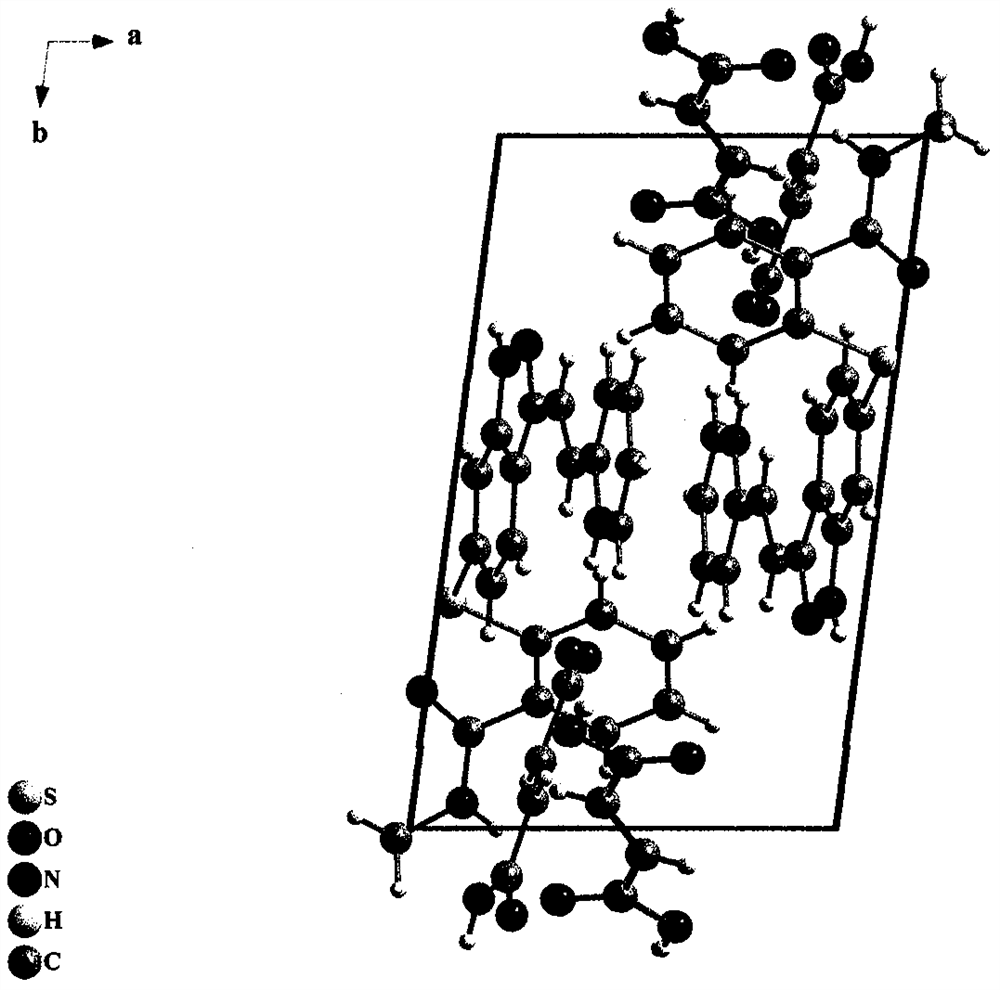
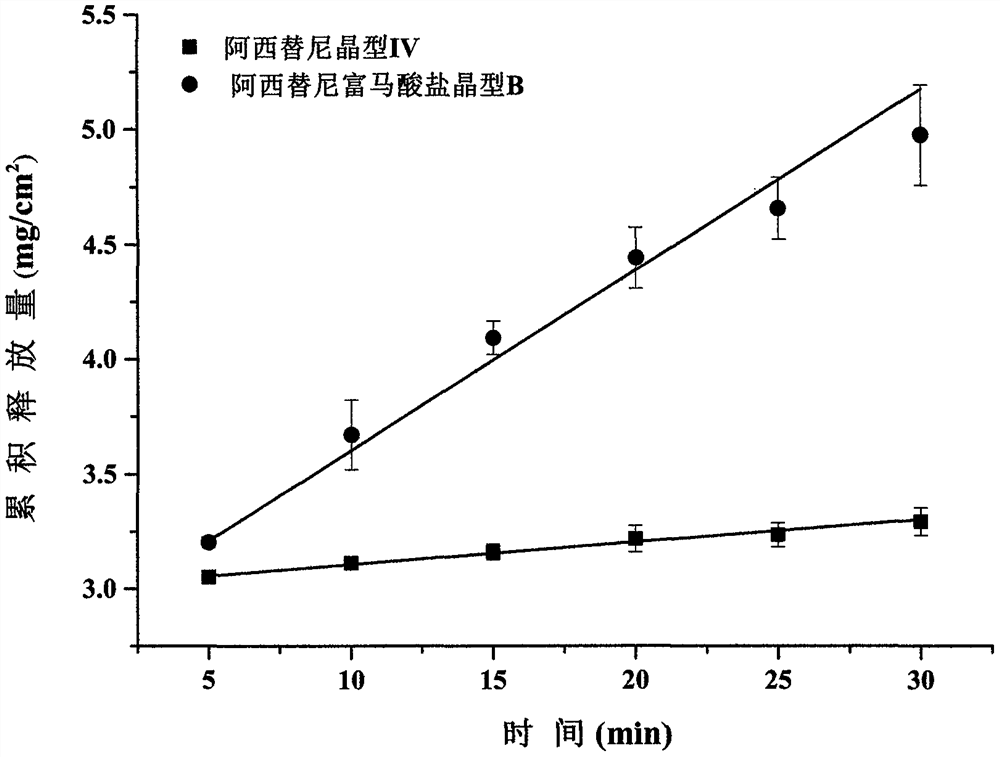
New crystal form of axitinib fumarate and preparation method thereof
InactiveCN112174933AImprove securityImprove oral bioavailabilityOrganic active ingredientsOrganic chemistry methodsBioavailabilityPowder diffraction
The invention discloses a new crystal form of axitinib fumarate and a preparation method thereof. In the new crystal form, the molar ratio of axitinib to fumaric acid is 1: 1.5, and the X-ray powder diffraction pattern of the crystal form has characteristic peaks when the 2 theta value is 7.1 + / -0.2 degrees, 12.5 + / -0.2 degrees, 15.1 + / -0.2 degrees, 17.4 + / -0.2 degrees and 23.4 + / -0.2 degrees. Thecrystal form preparation method provided by the invention is simple in process, easy in crystallization process control, good in reproducibility and suitable for industrial production. The new crystal form of the axitinib fumarate is remarkably improved in the aspects of light stability and dissolution property, and is beneficial to improving the safety and oral bioavailability of axitinib.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Eutectic crystal of lenvatinib and benzoic acid, and preparation method thereof
ActiveCN111793027AImprove stabilityImprove efficiencyOrganic chemistry methodsAntineoplastic agentsSolubilityLenvatinib Mesylate
The invention discloses a lenvatinib and benzoic acid eutectic crystal and a preparation method thereof, wherein in the eutectic crystal, the molar ratio of lenvatinib to benzoic acid is 1:1, and theeutectic X-ray powder diffraction pattern has characteristic peaks when the 2theta values are 6.4+ / -0.2 degrees, 7.4+ / -0.2 degrees, 12.5+ / -0.2 degrees, 14.7+ / -0.2 degrees, 16.7+ / -0.2 degrees, 17.7+ / -0.2 degrees and 23.8+ / -0.2 degrees. According to the invention, the eutectic preparation method is simple in process, easy to control the crystallization process, good in reproducibility and suitable for industrial production; and compared with lenvatinib mesylate, the eutectic crystal of the invention has low hygroscopicity, high dissolution rate and high apparent solubility, and provides a material basis for improving the stability and oral absorption efficiency of lenvatinib and reducing the process threshold and cost of a preparation.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Method for extracting naphthalin from heavy C10 aromatic solvent oil by integral device
InactiveCN107814678ALow in naphthaleneSimple processHydrocarbonsCrystallisation purification/separationVacuum pumpingAromatic solvent
The invention discloses a method for extracting naphthalin from heavy C10 aromatic solvent oil by an integral device. The integral device comprises a multistage axial stirring crystallizer device, a filter device and a collecting device which are sequentially connected from top to bottom. The method comprises the following steps of adding the raw material of heavy C10 aromatic solvent oil from a feeding opening; controlling the feeding speed and the rotating speed of the stirring shaft; controlling the crystallization and the filtering to be at the same temperature through constant temperaturemedia; performing vacuum pumping on the filter device so that the filter device is in a vacuum state; downwards flowing the raw materials into the filter device from the multistage axial stirring crystallizer device; performing vacuum filtering in the filter device; finally, collecting filter liquid through the collecting device; collecting filter cake through the filter device, wherein the filter cake is the extracted naphthalin. The method has the advantages that the process is simple; the continuous operation can be realized; the used device is integral; the crystallization and the filtering can be conveniently controlled to be at the same temperature; after the condition of optimization separation, the naphthalin content in the light C10 aromatic solvent oil is lower than 3 percent; the content and the yield of naphthalin are as high as 99.54 percent and 97.17 percent or higher.
Owner:NANJING NORMAL UNIVERSITY
Penciclovir and 3,5-dihydroxybenzoic acid eutectic crystal and preparation method thereof
ActiveCN110627793AImprove apparent solubilityImprove bioavailabilityOrganic chemistry methodsAntiviralsSolubilityDissolution
The invention discloses a penciclovir and 3,5-dihydroxybenzoic acid eutectic crystal and a preparation method thereof. In the penciclovir and 3,5-dihydroxybenzoic acid eutectic crystal, the molar ratio of penciclovir to 3,5-dihydroxybenzoic acid is 1:1. The preparation method of the penciclovir and 3,5-dihydroxybenzoic acid eutectic crystal is simple in process, the crystallization process is easyto control, the reproducibility is good, and the penciclovir and 3,5-dihydroxybenzoic acid eutectic crystal is suitable for industrial production. Compared with penciclovir, the penciclovir and 3,5-dihydroxybenzoic acid eutectic crystal has the advantages that the dissolution rate is higher, the apparent solubility is higher, the bioavailability of the penciclovir is favorably improved, and a material basis is provided for successfully developing oral preparations and liquid preparations of penciclovir.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
A kind of pearl paste fatty acyl glutamate sodium composition and facial cleanser
ActiveCN109363960BNo pollution in the processEasy accessCosmetic preparationsToilet preparationsBiotechnologyMonosodium glutamate
The invention discloses a pearl paste-like sodium fatty acylglutamate composition, which is prepared from the following components in mass percentage: sodium fatty acylglutamate 6-40%, disodium fatty acylglutamate 10‑40%, fatty acylglutamic acid 3‑20%, polyol 5‑30%, salt 0‑5%, deionized water 10‑55%, preservative 0.1‑1%. The invention also provides a facial cleanser. The composition of the present invention can effectively avoid the problems of uneven texture and large dust existing in the prior art during production, and its formula construction is relatively simple, the raw materials are convenient to obtain, the production is convenient, there is no dust pollution, and it is easy to thicken. The advantage of crystallization is easy to control.
Owner:GUANGZHOU BAFEORII CHEM
Epalrestat-metformin salt as well as preparation method and application thereof
ActiveCN113336718AIncrease dissolution rateImprove solubilityMetabolism disorderOrganic compound preparationSolubilityFluid phase
The invention discloses epalrestat-metformin salt as well as a preparation method and application thereof. The molecular formula of the epalrestat-metformin salt is C19H24N6O3S2, and the molecular weight of the epalrestat-metformin salt is 448.6; the epalrestat-metformin salt is in a crystalline state. The preparation method of the epalrestat-metformin salt comprises the following steps: dissolving epalrestat and metformin in a solvent according to a molar ratio of 1: 0.8-1: 1.2 to obtain a mixture; reacting and crystallizing the mixture for 12-48 hours under the condition of 15-60 DEG C; and carrying out solid-liquid phase separation on the obtained product, and drying to obtain the epalrestat-metformin salt. Compared with a metformin single product, the epalrestat-metformin salt form provided by the invention has the advantages that the strong hygroscopicity is greatly improved, the solubility is greatly improved compared with an epalrestat single product, meanwhile, the preparation method is simple to operate, the crystallization process is easy to control, and the reproducibility of the salt form is good.
Owner:TIANJIN UNIV
Crystal form of dapagliflozin, preparation method, and applications thereof
ActiveCN109705076AGood for mass manufacturingThe crystallization rate is stableOrganic active ingredientsOrganic chemistryOrganic solventCrystal structure
The invention discloses a crystal form of dapagliflozin and preparation method thereof. The dapagliflozin is stirred and dissolved with 1,2-propylene glycol in an organic solvent; then a low-polar solvent is added, and cooling crystallization is carried out at certain temperature; the mixture then is filtered to obtain a solid; the solid is subjected to pressure-reduced vacuum drying to prepare acrystal form compound of dapagliflozin, which has high purity and has obvious structural features of the crystal. The crystal form compound of dapagliflozin has good thermal stability and allows large-scale production. The crystal form compound and a composition thereof are mainly used for treatment on diabetes type II.
Owner:JIANGSU SUZHONG PHARM GRP CO LTD
Metformin-tolbutamide new salt type and preparation method and medical application thereof
InactiveCN110128305AGood hygroscopicityImprove stabilityOrganic compound preparationMetabolism disorderSolubilitySolvent
The invention discloses a metformin-tolbutamide new salt type and a preparation method and medical application thereof. The preparation method comprises the following steps: dissolving metformin and tolbutamide in an appropriate solvent, reacting, crystallizing, filtering and drying. The preparation method has simple operation, good reproducibility and easy control. The hygroscopicity and stability of the metformin-tolbutamide new salt type are greatly improved compared with that of a metformin single product, the solubility of the metformin-tolbutamide new salt type is greatly improved compared with that of a tolbutamide single product, the preparation method is simple, the crystallization process is easy to control, and the metformin-tolbutamide new salt type has good repeatability.
Owner:TIANJIN UNIV
Ketorolac and 4-pyridine carboxamide eutectic crystal and preparation method thereof
PendingCN114380833AImprove solubilityReliable Pharmaceutical Active IngredientsOrganic active ingredientsAntipyreticO-Phosphoric AcidKetorolac
The invention belongs to the technical field of pharmaceutical co-crystals, and provides a ketorolac and 4-pyridinecarboxamide co-crystal and a preparation method thereof.The prepared ketorolac and 4-pyridinecarboxamide co-crystal is radiated through Cu-K alpha, and an X-ray diffraction pattern expressed by 2 theta has characteristic peaks at the positions of 5.7 + / -0.2 degrees, 6.6 + / -0.2 degrees, 11.5 + / -0.2 degrees, 13.4 + / -0.2 degrees, 15.7 + / -0.2 degrees, 17.5 + / -0.2 degrees, 18.3 + / -0.2 degrees and 27.4 + / -0.2 degrees; according to the ketorolac and 4-pyridine carboxamide eutectic crystal prepared by the invention, the yield is greater than 93%, the purity is higher than 99.80%, the preparation method is simple, the crystallization process is easy to control, and the reproducibility is good; compared with the existing ketorolac crystal form or ketorolac standard substance, the ketorolac crystal form or ketorolac standard substance has higher solubility in a phosphoric acid buffer solution with pH of 6.8.
Owner:LUNAN PHARMA GROUP CORPORATION
Co-crystal of abiraterone acetate and trans-aconitic acid, and preparation method, pharmaceutical composition and application thereof
ActiveCN113197865AEasy to operateThe crystallization process is easy to controlPowder deliveryOrganic active ingredientsAbirateronePowder diffraction
The invention relates to a co-crystal of abiraterone acetate and trans-aconitic acid, a preparation method thereof, a pharmaceutical composition and application of the co-crystal. An X-ray powder diffraction pattern of the eutectic crystal of abiraterone acetate and trans-aconitic acid has characteristic peaks when diffraction angles 2 theta are 6.7 degrees, 7.3 degrees, 7.6 degrees, 13.6 degrees, 14.0 degrees, 15.0 degrees, 17.1 degrees, 17.9 degrees, 18.5 degrees, 19.5 degrees and 24.9 degrees. Compared with abiraterone acetate in the prior art, the eutectic crystal of abiraterone acetate and trans-aconitic acid provided by the invention has the advantage that the drug solubility, dissolution performance, in-vivo drug absorption and the like are remarkably improved.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com