Co-crystal of abiraterone acetate and trans-aconitic acid, and preparation method, pharmaceutical composition and application thereof
A technology of abiraterone acetate and trans-aconitic acid is applied in the directions of drug combination, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., to achieve high bioavailability, obvious improvement, and improved solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
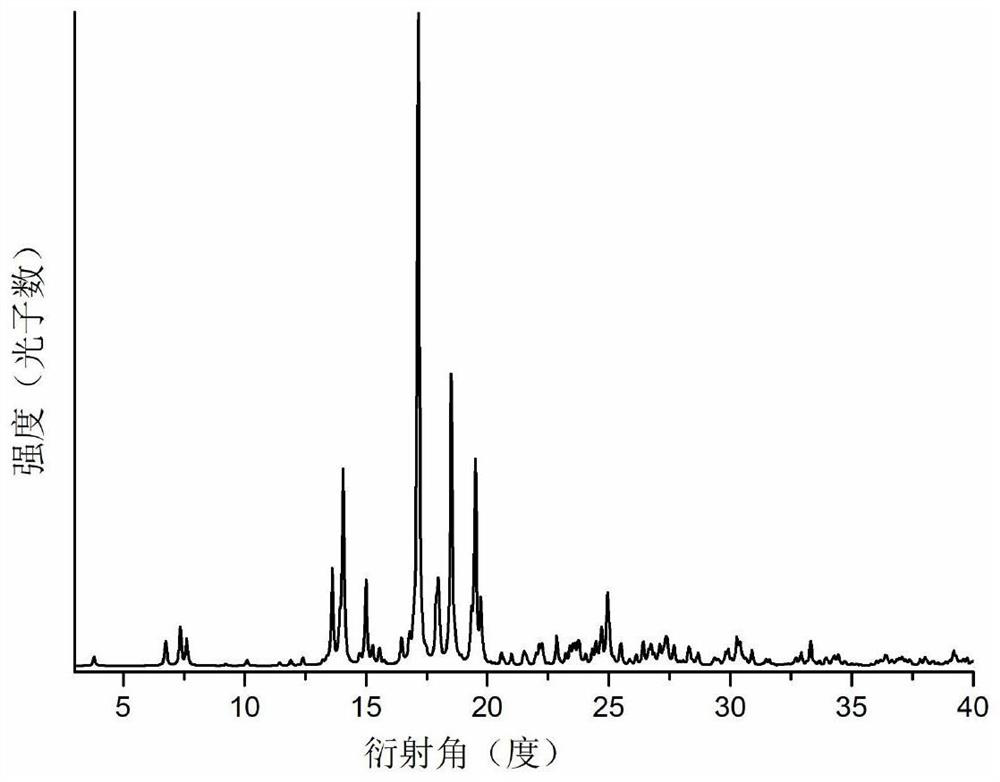
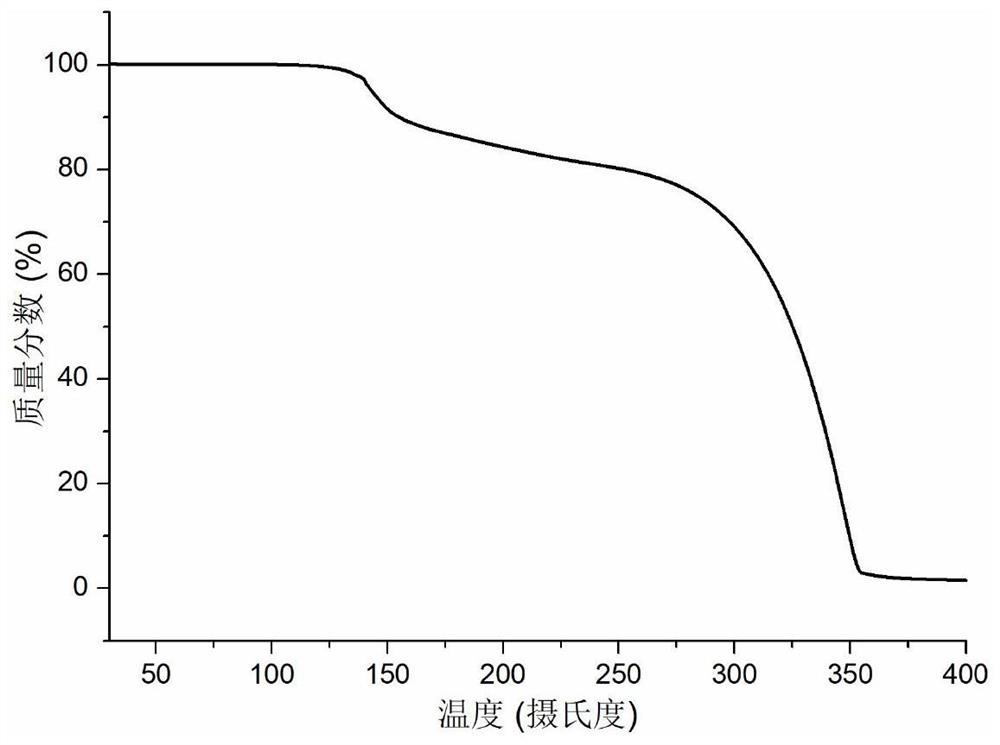
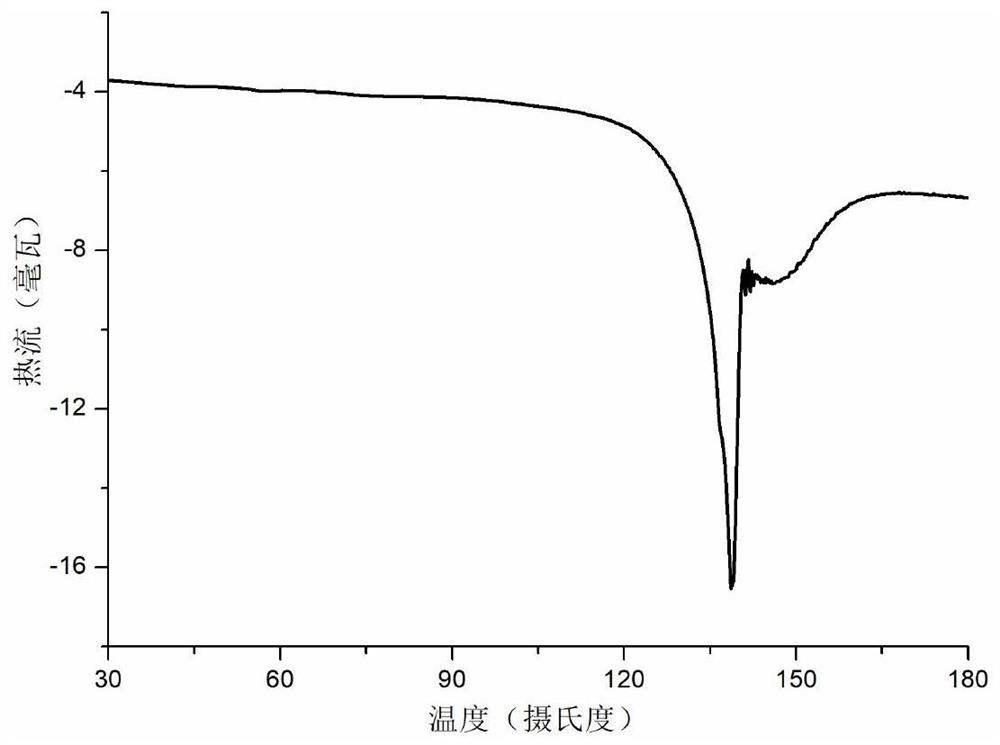
Image
Examples
Embodiment 1
[0053] Abiraterone acetate 0.1mmol and trans-aconitic acid 0.05mmol are added in 4 milliliters of ethanol according to stoichiometric ratio 2:1, after dissolving completely, slowly volatilize at room temperature again, obtain abiraterone acetate and trans-aconitic acid Co-crystal of head acid.
Embodiment 2
[0055] Add 0.1mmol of abiraterone acetate and 0.05mmol of trans aconitic acid into 4 milliliters of methanol according to the stoichiometric ratio of 2:1, after completely dissolving, volatilize slowly at 50 degrees Celsius to obtain abiraterone acetate and trans aconitic acid Co-crystal of aconitic acid.
Embodiment 3
[0057] Abiraterone acetate 0.1mmol and trans-aconitic acid 0.05mmol are added in 4 milliliters of acetone according to stoichiometric ratio 2:1, after dissolving completely, slowly volatilize under the condition of 50 degrees Celsius again, obtain abiraterone acetate and trans-aconitic acid Co-crystal of aconitic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com