Crystal form of dapagliflozin, preparation method, and applications thereof
A technology of dapagliflozin and crystal form, which is applied in the field of compound crystal form, can solve problems such as difficulty in stirring, large particles, large particle size and hardness, etc., and achieve the effect of easy crystallization process and stable crystallization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
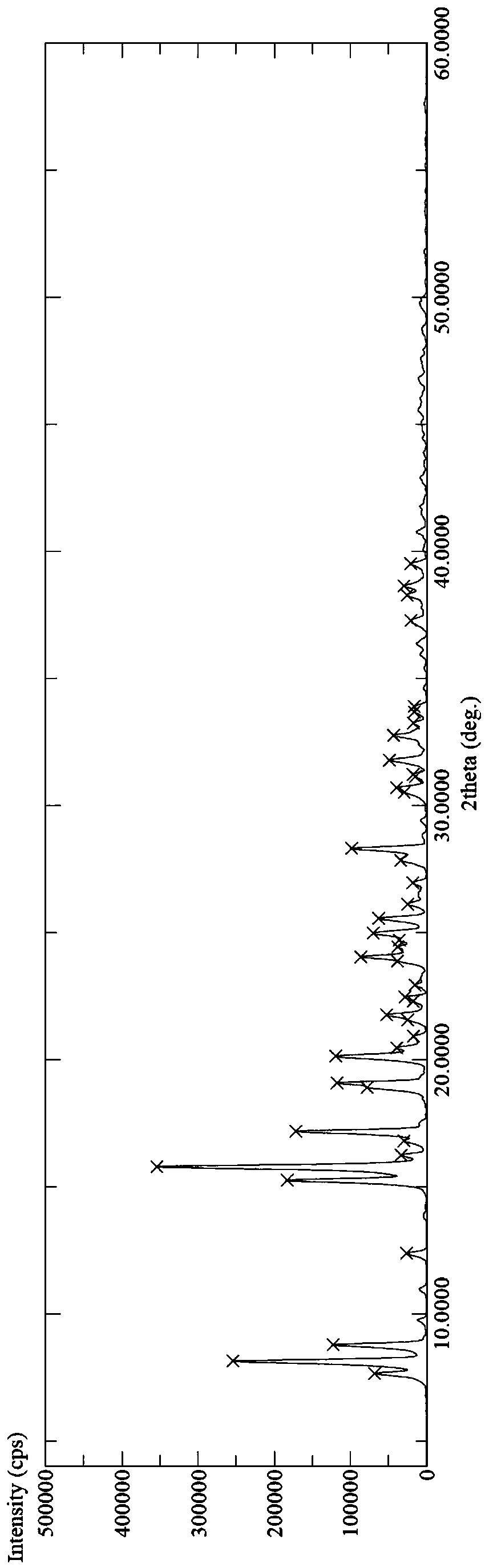
Image
Examples
Embodiment 1
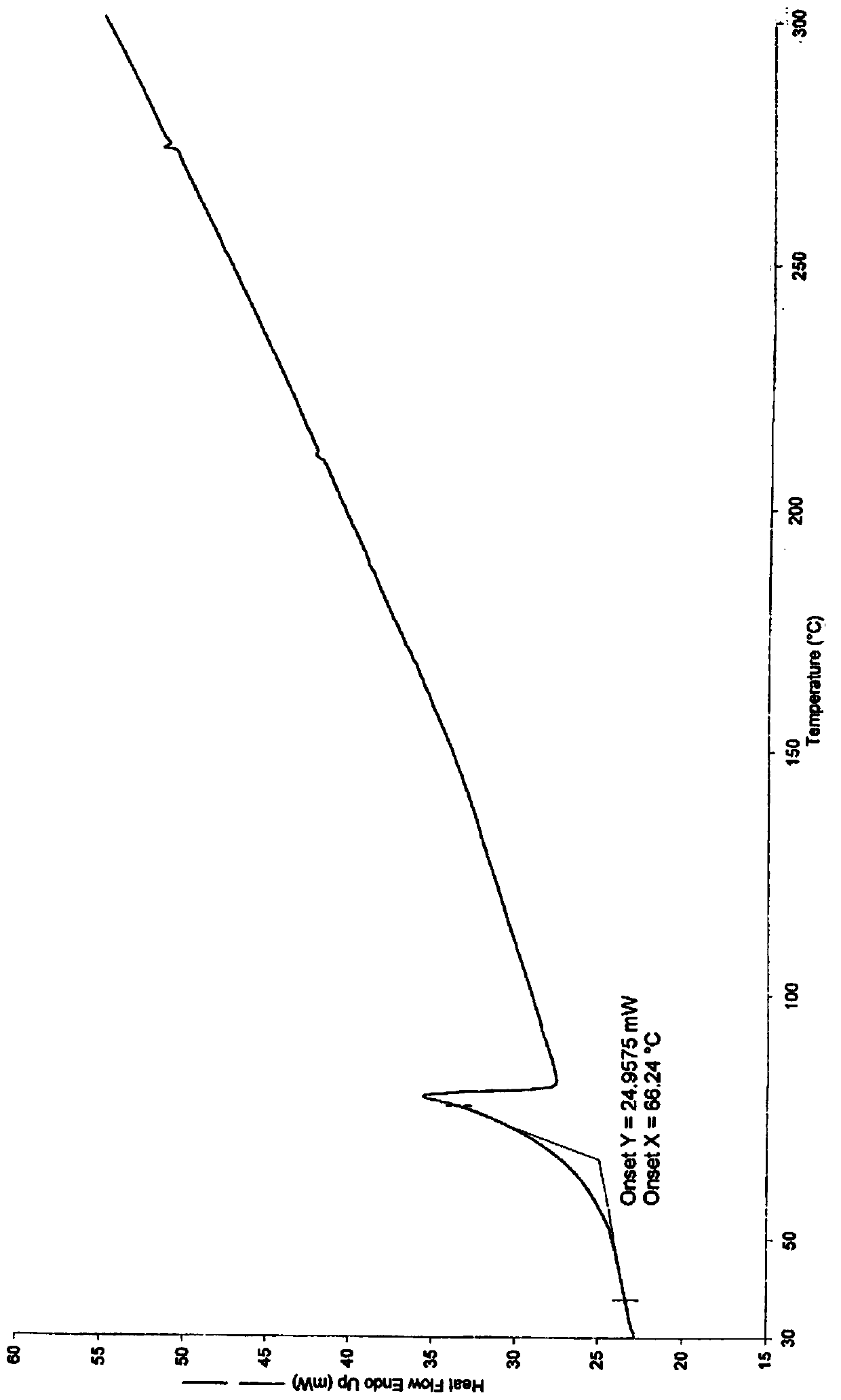
[0039] Take 4.06g (9.94mmol) of Daxigliflozin and add it to 40ml of isopropyl acetate, stir at 29°C, add 0.80g (10.51mmol) of 1,2-propanediol, stir at a speed of 100 rpm, after 1h, Add 40ml of cyclohexane. After the addition of cyclohexane, the solvent system becomes turbid, and the solid crystallizes. Continue to stir for 30 minutes, filter with suction, wash the filter cake with a small amount of cyclohexane, and dry it in vacuum at 30°C for 24 hours to obtain 4.00g of white solid powder. (7.95 mmol), which is the crystal form compound Ic of dapagliflozin; the yield is 80.0% (molar yield, calculated based on the amount of dapagliflozin added, the same below), the purity is 99.9%, and the melting point is 76°C.
[0040] The detection method of purity is HPLC method, inertsil ODS-SP 5um 4.6×250mm column, flow rate 1.0ml / min, detection at 220nm, methanol-water (75:25) as mobile phase, area normalization method calculation, the same below .
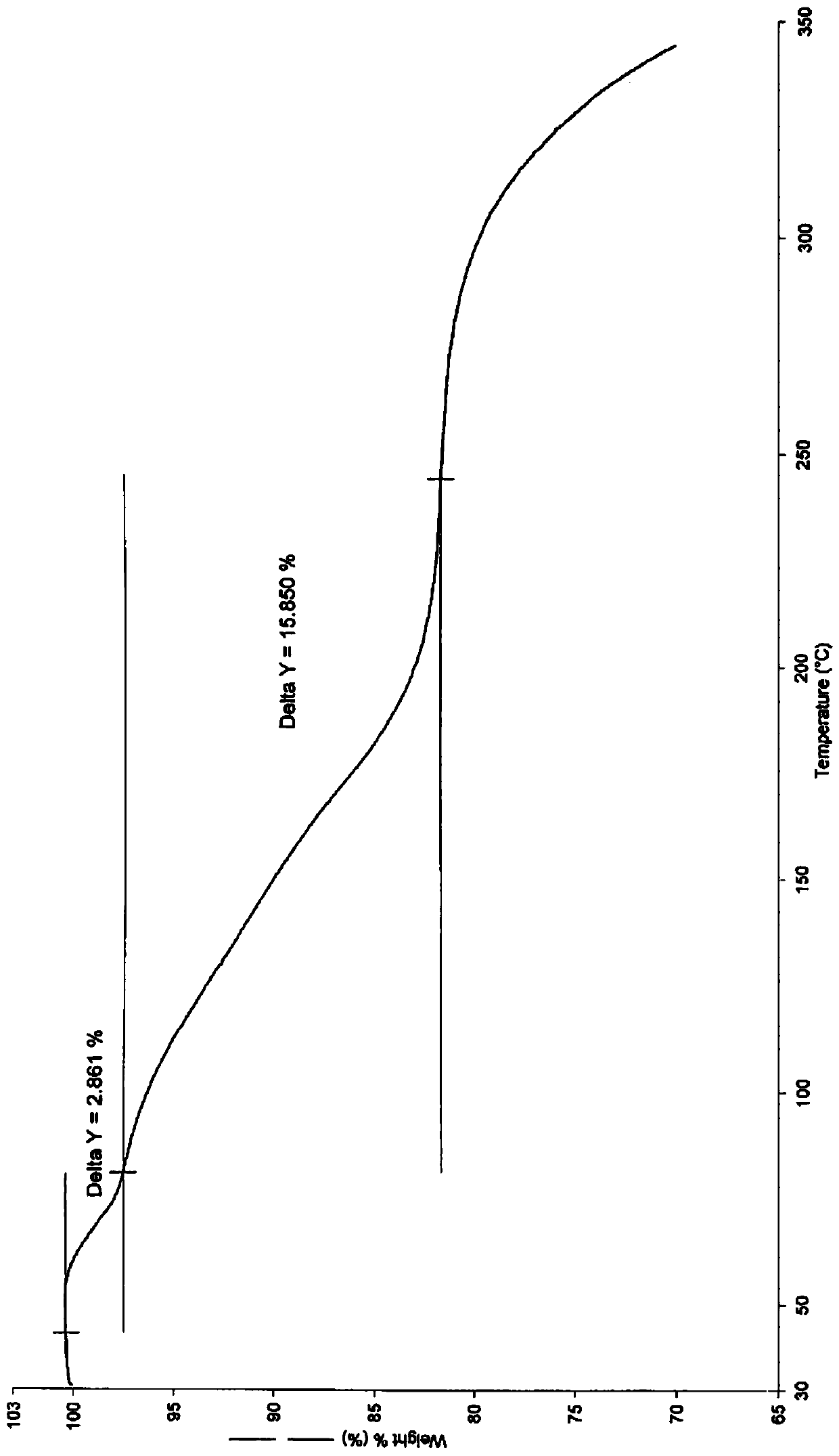
[0041] Using a thermogravimetric an...
Embodiment 2
[0048] Take 4.05g (9.91mmol) of Daxigliflozin and add it to 40ml of isopropyl acetate, stir at 0°C, add 0.81g (10.60mmol) of 1,2-propanediol, stir at a speed of 100 rpm, after 1h, Add 40ml of cyclohexane. After the addition of cyclohexane, the solvent system becomes turbid, and the solid crystallizes. Continue to stir for 3h, filter with suction, wash the filter cake with a small amount of cyclohexane, and vacuum dry at 30°C for 24h to obtain 4.28g of white solid powder (8.51mmol), yield 87.0%, purity 99.9%, melting point 76 ° C, because the preparation method is similar to Example 1, and the melting point is consistent, indicating that the same crystal form of dapagliflozin compound was obtained as in Example 1, the same below.
Embodiment 3
[0050] Add 4.03g (9.86mmol) of Daxigliflozin into 40ml of methyl tert-butyl ether, stir at 0°C, add 0.78g (9.86mmol) of 1,2-propanediol, stir at 100 rpm, 1h Finally, add 40ml of n-heptane. After the addition of n-heptane is completed, the solvent system becomes turbid, and the solid crystallizes. Continue to stir for 60 minutes, filter with suction, wash the filter cake with a small amount of n-heptane, and dry it in vacuum at 30°C for 24 hours to obtain a white solid powder 4.48g (8.91mmol), yield 90.4%, purity 99.9%, melting point 76°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com