Organic fluorescent sensing material with high-sensitivity fluorescent response to several explosives, and preparation method application of organic fluorescent sensing material
A fluorescence sensing and fluorescence response technology, applied in luminescent materials, fluorescence/phosphorescence, organic chemistry, etc., can solve the problems of unfavorable detection, single detection object, low sensitivity, etc., and achieve high selectivity, high sensitivity, and high stability. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
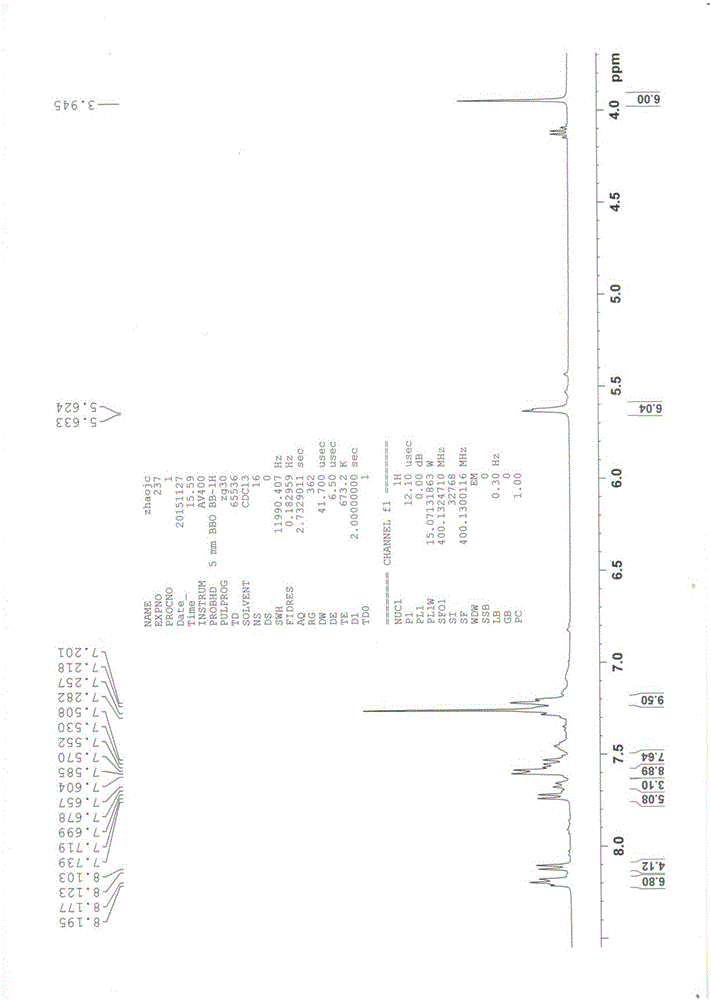
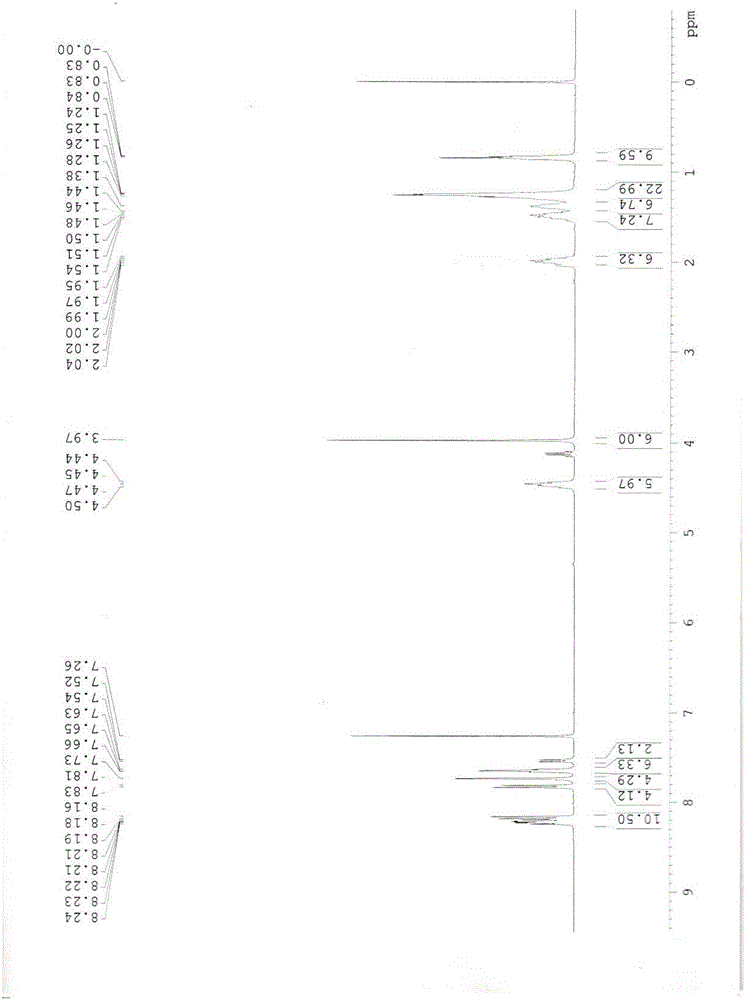
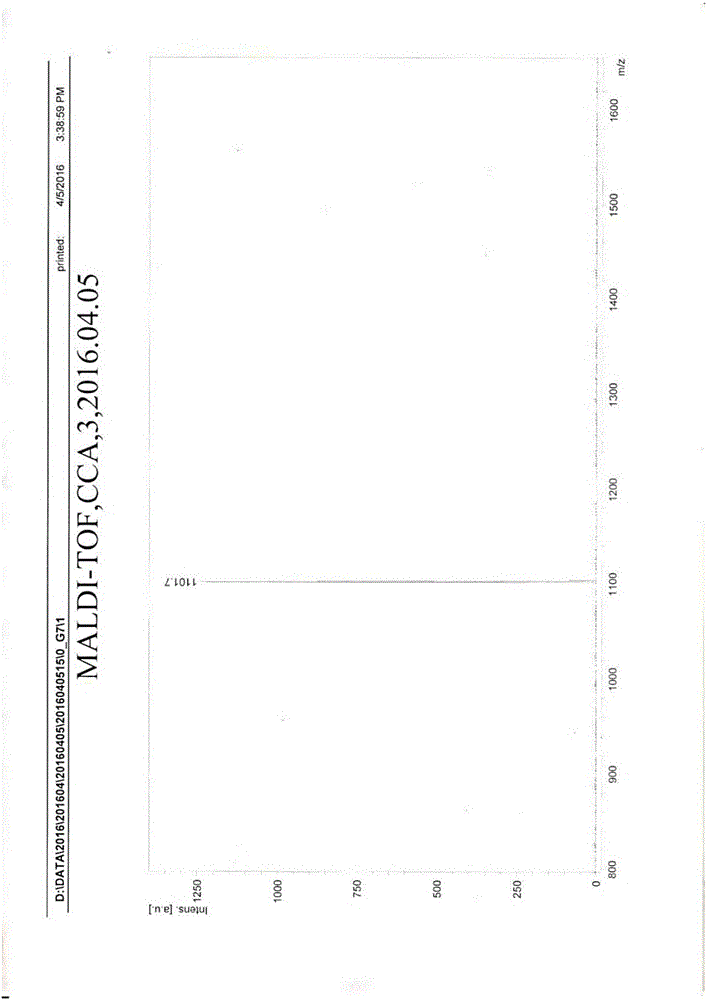
Image
Examples
preparation example Construction
[0077] As mentioned above, the present invention discloses a method for preparing an organic fluorescent sensing material with fluorescent response to several types of explosives. The method comprises the following steps: (1) firstly synthesizing the carbazole derivative, (2) Then, in the mixture of good solvent and poor solvent, the organic fluorescent sensing material is obtained by self-assembly.
[0078] In a preferred embodiment of the present invention, the carbazole derivative of n=3 in the preparation formula (I), said step (1) specifically comprises:
[0079] (1a) the compound shown in the formula (II) reacts with RX ' to prepare the compound shown in the formula (III);
[0080]
[0081] X in formula (II) and formula (III) is the same or different, and is independently selected from halogen (such as Br, I); X' in RX' is selected from halogen (such as Br, I); Formula (III) and The definition of R in RX' is the same as formula (I);
[0082] (1b) compound shown in f...
Embodiment 1
[0127] Preparation has the following molecular 1 R is a straight-chain octyl group, R' is a 4-methoxyacylphenyl group, and n is a carbazole derivative of 3, and its preparation method is as follows:
[0128]
[0129] (1) Dissolve 1 g of 2,7-dibromocarbazole in 30 ml of N,N-dimethyl-formamide (DMF) solution, place the above solution in an ice bath at 0°C, and slowly add 1.2 The equivalent of 74 mg of sodium hydride solid was continuously stirred for half an hour, then 1.5 equivalent of 1-bromooctane was slowly added, and after reacting overnight at room temperature, the product was obtained by column chromatography.
[0130] (2) Get 500 mg of the product obtained in step (1), add 20 ml of 1,4-dioxane solution, add 5 equivalents of bisvaleryl diboron, 14 equivalents of potassium acetate, 10% equivalents of [1,1 '-bis(diphenylphosphino)ferrocene]palladium dichloride was reacted for 6 hours at 80°C under the protection of argon, and the product (TM-1) was obtained by column chr...
Embodiment 2
[0135] Preparation has the following molecule 2 R is an isobutyl group, R' is a 4-methoxyacylphenyl group, and n is a carbazole derivative of 3, and its preparation method is as follows:
[0136]
[0137] (1) Add 14.2g p-toluenesulfonyl chloride (TsCl) to 150ml dichloromethane, add 824mg DMAP and 15g triethylamine under argon protection and 0°C ice bath, after deoxygenation, add 5g iso Butanol was dissolved in 50ml of dichloromethane, slowly added to the above solution, after the reaction was stirred overnight, a saturated sodium bicarbonate solution was added to quench the reaction, after extraction with 100ml×3 dichloromethane, the organic phases were combined, and anhydrous sulfuric acid was added After sodium drying, the product was spin-dried.
[0138] (2) Dissolve 1 gram of 2,7-dibromocarbazole in 30 ml of DMSO, slowly add 9 equivalents of 1.55 g of potassium hydroxide solid, continue stirring for half an hour, slowly add 1.5 equivalents of 1.05 g of the step The produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com