Sulfonylurea compound and metformin salts, preparation method and application
A technology for metformin and a compound, applied in the field of chemical synthesis and crystallization, can solve the problems of poor pharmaceutical properties, low solubility, large oral dose and the like, and achieve the effects of easy control of the crystallization process, improved hygroscopic stability, and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
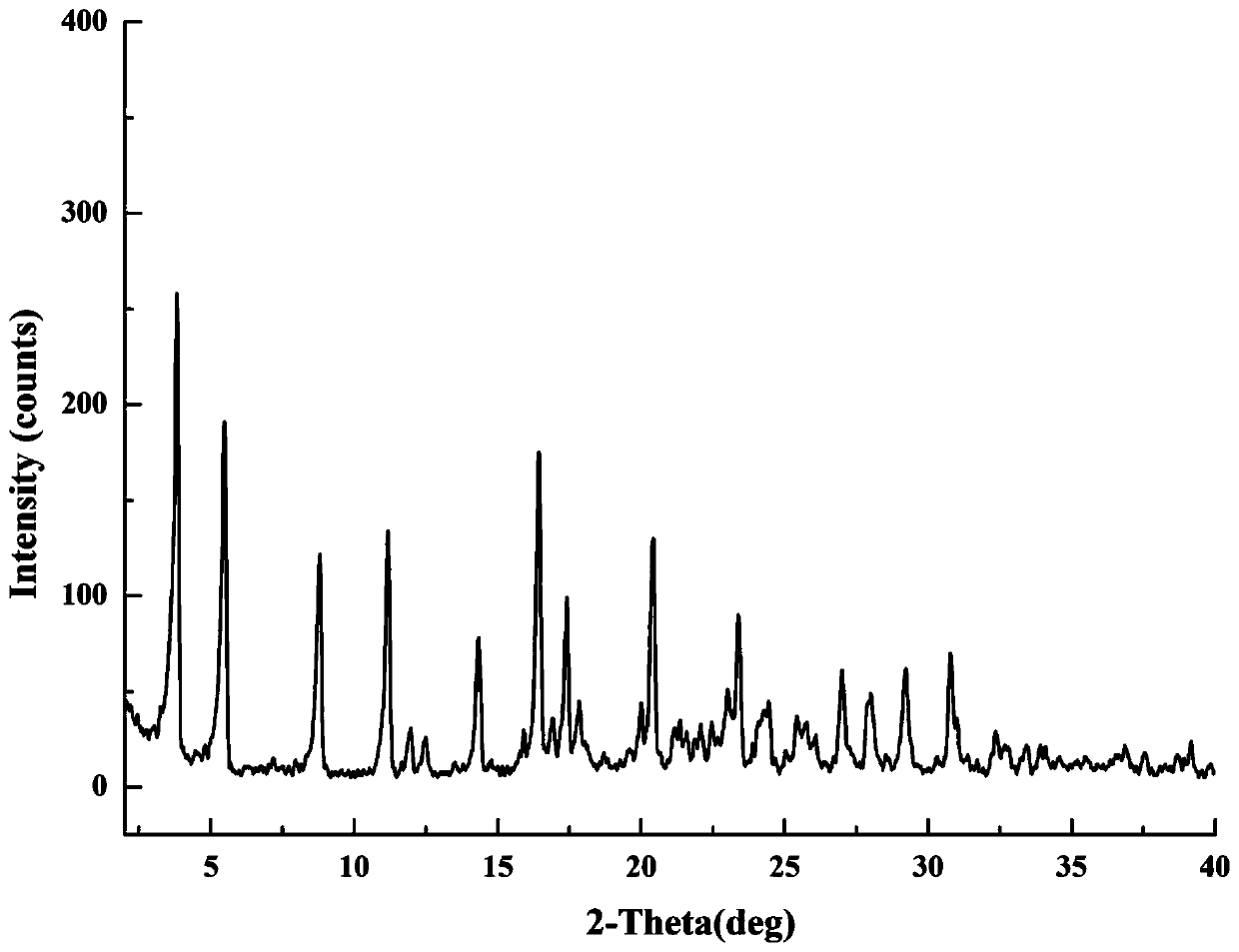
[0052] Take 52.7mg of gliquidone and 13.0mg of metformin (the molar ratio is 1:1) and put them in a 4mL sample bottle, add 2mL of acetonitrile, sonicate, make it dissolve and be in a supersaturated state, and react and crystallize at room temperature for 24 hours. The suspension is centrifuged, the supernatant is discarded, and the centrifuged solid is dried in a 40°C forced air oven for 3 hours to obtain the gliquidone-metformin salt. The diffraction angle represented by the 2θ angle in the XRPD result is 3.82±0.20°, 5.48±0.20°, 8.80±0.20°, 11.18±0.20°, 14.34±0.20°, 16.42±0.20°, 17.40±0.20°, 17.84±0.20°, 20.42±0.20°, 23.38±0.20°, There are characteristic peaks at 27.02±0.20°, 28.00±0.20°, 29.22±0.20°, 30.78±0.20°, such as figure 1 . The chemical structural formula is as follows:
[0053]
Embodiment 2
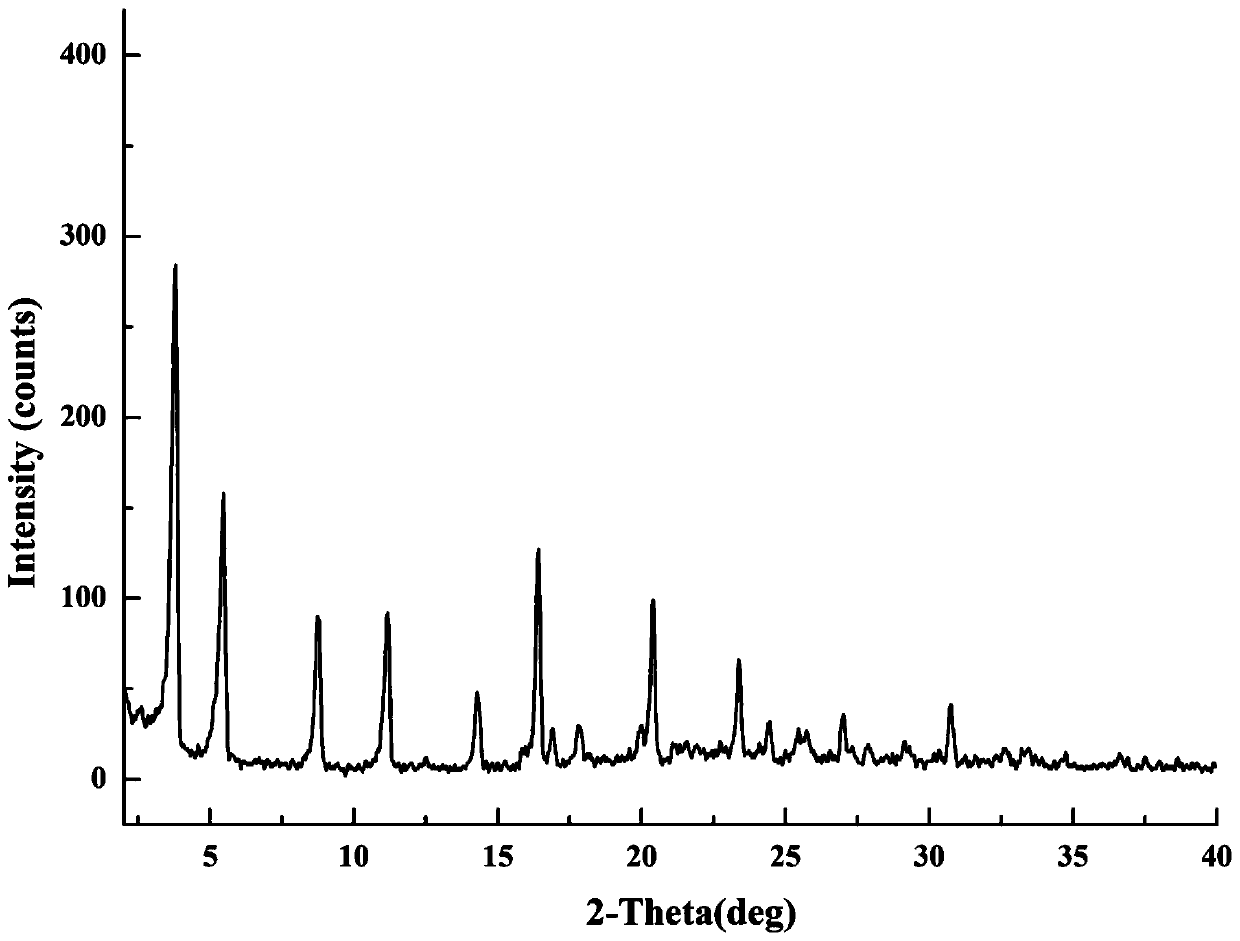
[0055] Take 52.7mg of gliquidone and 13.0mg of metformin (the molar ratio is 1:1) and place them in a 4mL sample bottle, add 2mL of ethanol, ultrasonicate, make it decompose and be in a supersaturated state, and react and crystallize at room temperature for 24 hours. The suspension was centrifuged, the supernatant was discarded, and the centrifuged solid was dried in a 40°C forced air oven for 3 hours to obtain the gliquidone-metformin salt. The diffraction angle represented by the 2θ angle in the XRPD result was at 3.82±0.20°, 5.48±0.20°, 8.80±0.20°, 11.18±0.20°, 14.34±0.20°, 16.42±0.20°, 17.40±0.20°, 17.84±0.20°, 20.42±0.20°, 23.38±0.20°, There are characteristic peaks at 27.02±0.20°, 28.00±0.20°, 29.22±0.20°, 30.78±0.20°, such as figure 2 .
Embodiment 3
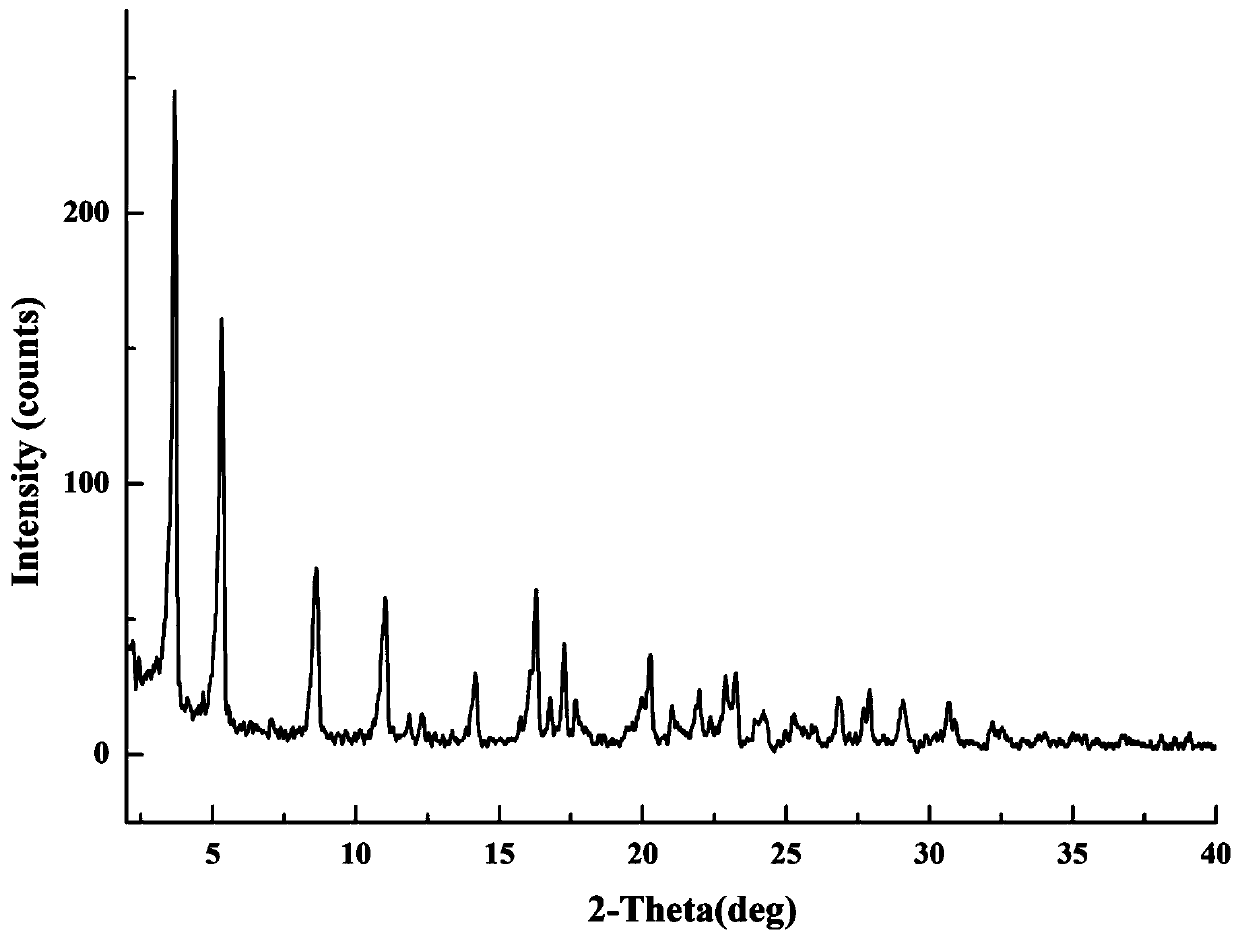
[0057] Take 52.7mg of gliquidone and 12.0mg of metformin (molar ratio is 1:0.92) into a 4mL sample bottle, add 2mL of acetone, sonicate to dissolve and become supersaturated, react and crystallize at 40°C for 12 hours, Centrifuge the suspension, discard the supernatant, and dry the centrifuged solid in a forced air oven at 60°C for 1 hour to obtain the gliquidone-metformin salt. The diffraction angle expressed by the 2θ angle in the XRPD result is At 3.82±0.20°, 5.48±0.20°, 8.80±0.20°, 11.18±0.20°, 14.34±0.20°, 16.42±0.20°, 17.40±0.20°, 17.84±0.20°, 20.42±0.20°, 23.38±0.20° , 27.02±0.20°, 28.00±0.20°, 29.22±0.20°, 30.78±0.20° have characteristic peaks, such as image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com