Epalrestat-metformin salt hydrate as well as preparation method and application thereof
A technology of metformin salt and epalrestat, which is applied in the field of medicine and chemical industry, can solve the problems of toxicity, poor physical and chemical properties, and no therapeutic effect of diabetes treatment, etc., to achieve reduced hygroscopicity, easy control of the crystallization process, and improved dissolution Effects of Rate and Solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
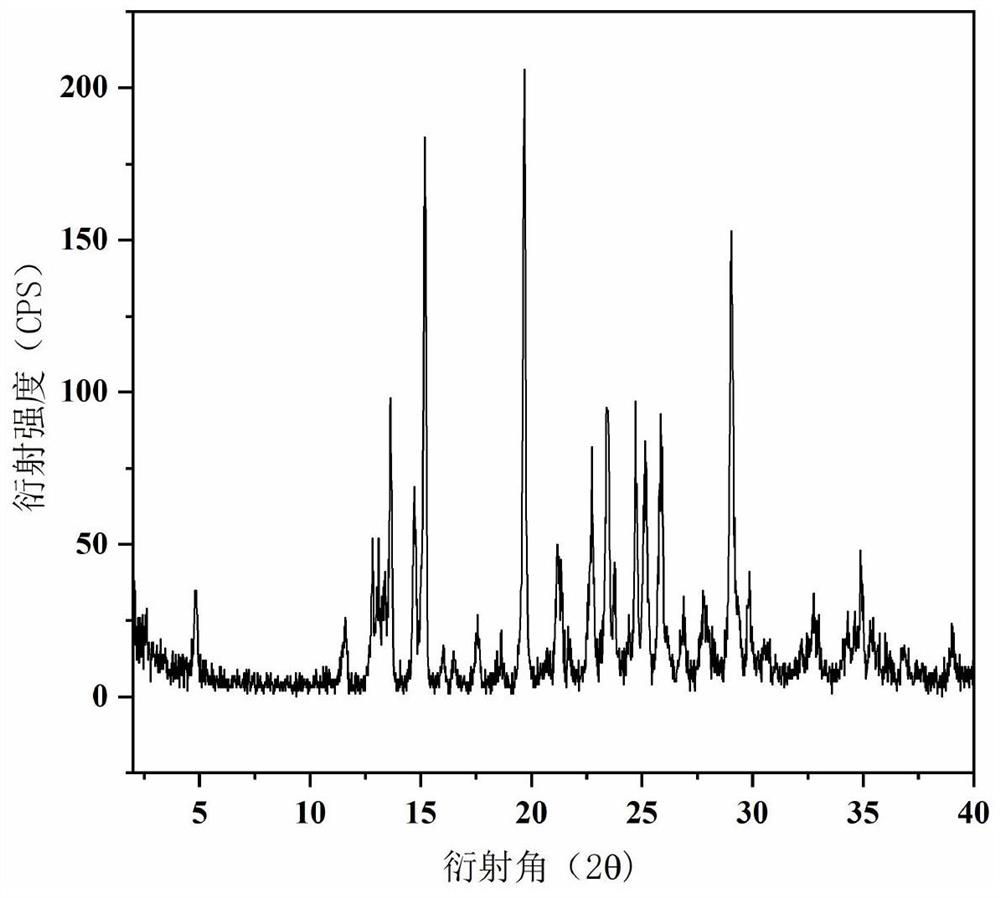
[0047] Take 32.0mg of epalrestat and 12.9mg of metformin (the molar ratio is 1:1) and place them in a 4mL sample bottle, add 2mL of acetone-water (v:v=4:1) mixed solvent, sonicate to dissolve and In a supersaturated state, react and crystallize at 30°C for 24 hours, centrifuge the suspension, discard the supernatant, and dry the centrifuged solid at room temperature for 3 hours to obtain epalrestat-metformin salt hydrate. Its XRPD results are as follows figure 1 .
[0048] figure 1 Prepare the XRD figure of product for embodiment 1, from figure 1 It can be seen that the diffraction angle is 2θ, which means that there are characteristic peaks at 4.93, 11.85, 12.96, 13.29, 13.90, 14.89, 15.34, 16.22, 17.88, 19.87, 21.45, 22.92, 23.77, and 25.43.
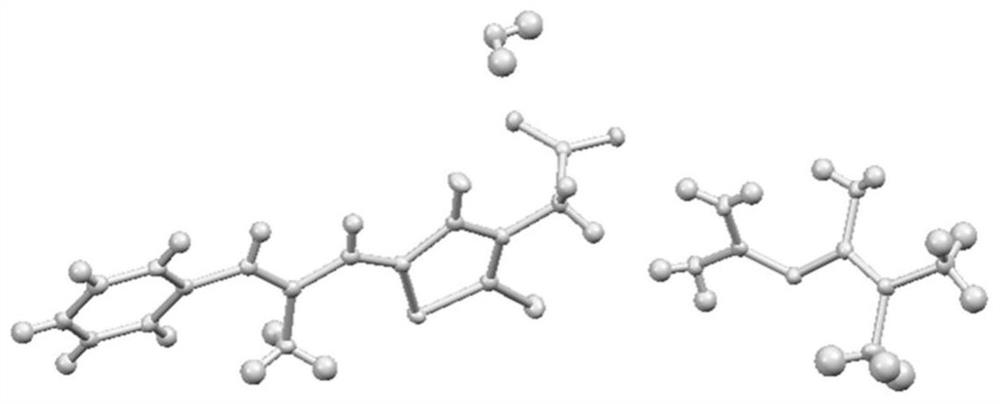
[0049] figure 2 Prepare the crystal structure figure of product for embodiment 1, from figure 2 It can be seen that the crystallographic characteristics of this product are: the bond length is a=7.8711 (4), b=8.3789 (4), c=18.34...
Embodiment 2
[0058] Take 32.0mg of epalrestat and 11.9mg of metformin (molar ratio is 1:0.92) and place them in a 4mL sample bottle, add 2mL of acetone-water (v:v=1:1) mixed solvent, sonicate to dissolve and In a supersaturated state, react and crystallize at 45°C for 12 hours, centrifuge the suspension, discard the supernatant, and dry the centrifuged solid in a blast drying oven at 40°C for 1 hour to obtain epalrestat- Metformin Salt Hydrate.
[0059] Carry out XRD test to the product that embodiment 2 obtains, test product has characteristic peak at 4.93, 11.85, 12.96, 13.29, 13.98, 14.89, 15.34, 16.22, 17.88, 19.87, 21.55, 22.92, 23.77, 25.43 at diffraction angle 2θ .
[0060] The product that embodiment 2 obtains is carried out crystallographic test, finds that its crystallographic feature is: bond length is a=7.8711 (4), b=8.3789 (4), c=18.3489 (8), and bond angle is α=77.437 ( 4), β=82.244(4), γ=66.896(4), V=1084.7(6).
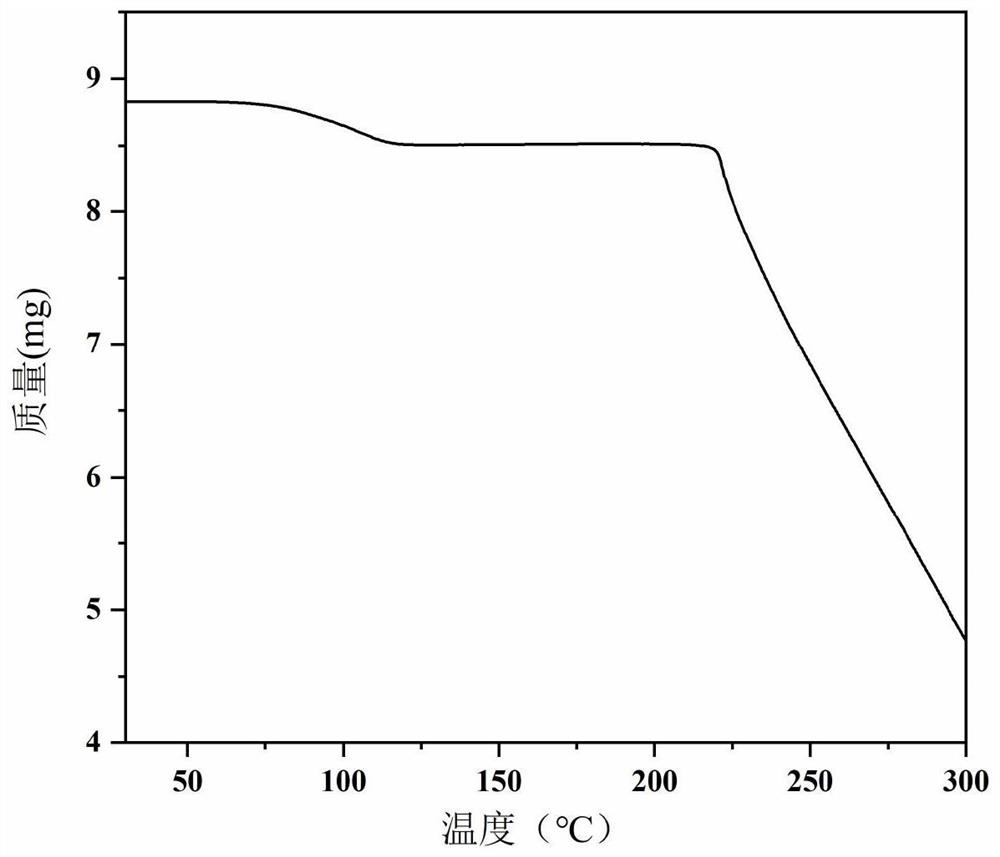
[0061] The product obtained in Example 2 was subjected to t...
Embodiment 3
[0065] Take 32.0mg of epalrestat and 15.5mg of metformin (molar ratio is 1:1.2) and place them in a 4mL sample bottle, add 2mL of acetone-water (v:v=4.5:1) mixed solvent, sonicate to dissolve and In a supersaturated state, react and crystallize at 60°C for 12 hours, centrifuge the suspension, discard the supernatant, and dry the centrifuged solid at room temperature 25°C for 12 hours to obtain epalrestat-metformin salt hydrate .
[0066] Carry out XRD test to the product that embodiment 3 obtains, test product has characteristic peak at 4.93, 11.85, 12.96, 13.29, 14.08, 14.89, 15.34, 16.22, 18.05, 19.87, 21.45, 23.02, 23.77, 25.43 at diffraction angle 2θ .
[0067] The product that embodiment 3 is obtained carries out crystallographic test, finds that its crystallographic feature is: bond length is a=7.8711 (4), b=8.3789 (4), c=18.3489 (8), and bond angle is α=77.437 ( 4), β=82.244(4), γ=66.896(4), V=1084.7(6).
[0068] The product obtained in Example 3 was subjected to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com