Epalrestat-metformin salt as well as preparation method and application thereof
A metformin and epalrestat technology, applied in the field of medicine and chemical industry, can solve the problems of poor physical and chemical properties, metformin instability, low solubility, etc., and achieve the effects of easy control of the crystallization process, reduced hygroscopicity, and reduced hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Take 32.0mg of epalrestat and 12.9mg of metformin (molar ratio is 1:1) into a 4mL sample bottle, add 2mL of ethanol, sonicate, dissolve and become supersaturated, react and crystallize at 15°C for 24 hours, The suspension is centrifuged, the supernatant is discarded, and the centrifuged solid is dried in a blast drying oven at 105° C. for 3 hours to obtain the epalrestat-metformin salt.
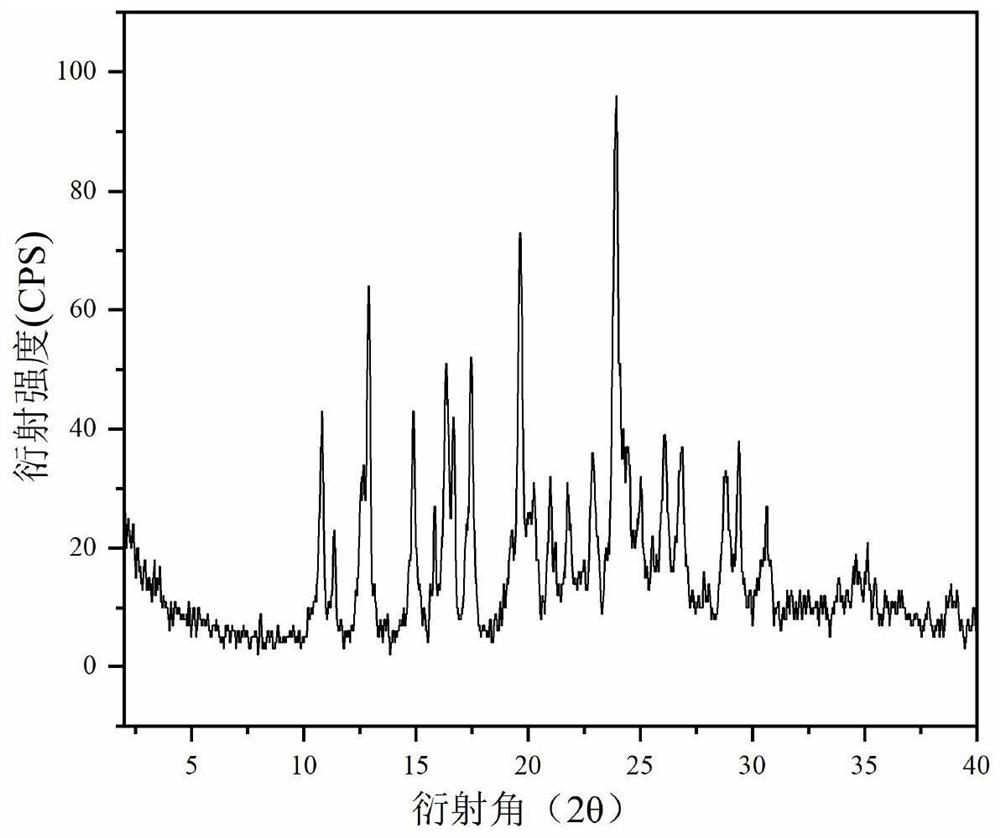
[0047] figure 1 Prepare the XRD figure of product for embodiment 1, from figure 1 It can be seen that the diffraction angle is 2θ, which means that there are features at 10.87, 11.34, 12.70, 12.90, 15.00, 16.29, 16.37, 16.67, 17.48, 19.27, 19.64, 20.28, 20.99, 21.81, 22.92, 24.00, 24.50, 25.05, 266.88, 2 peak.
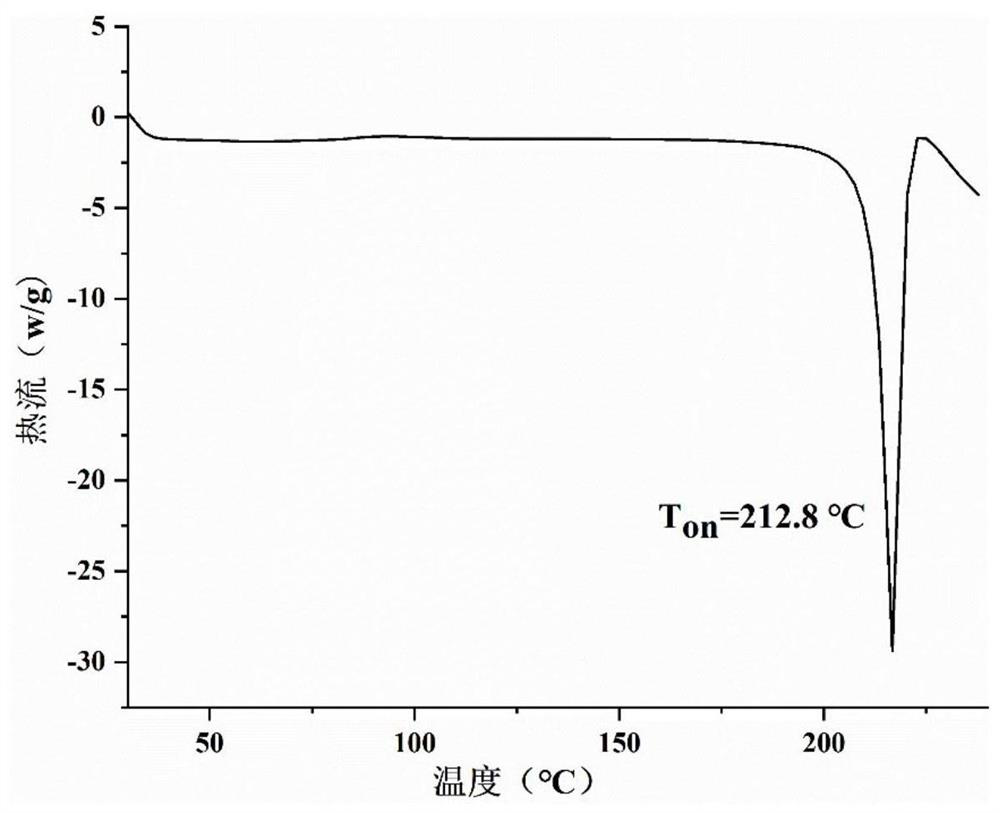
[0048] figure 2 Prepare the DSC figure that obtains product for embodiment 1, from figure 2 It can be seen that the differential scanning calorimetry spectrum of the epalrestat-metformin salt prepared in this example has a sharp endothermic peak at 215.7° C., which is the...
Embodiment 2
[0056] Take 32.0mg of epalrestat and 11.9mg of metformin (molar ratio is 1:0.92) into a 4mL sample bottle, add 2mL of isopropyl acetate, sonicate to make it dissolve and become supersaturated, react and crystallize at 60°C After 12 hours, the suspension was centrifuged, the supernatant was discarded, and the centrifuged solid was dried in a blast oven at 60° C. for 1 hour to obtain epalrestat-metformin salt.
[0057] Carry out XRD test to the product that embodiment 2 obtains, be 0.87,11.34,12.70,12.90,15.00,16.29,16.37,16.67,17.51,19.27,19.64,20.28,20.99,21.81,22.92,24.00,24.50, There are characteristic peaks at 25.10, 26.10, and 26.88. , so the product is the epalrestat-metformin salt.
[0058] The product obtained in Example 2 was tested by DSC. As can be seen from the test results, there is a sharp endothermic peak at 216.4° C., which is the melting point of epalrestat-metformin salt.
[0059] Embodiment 2 adopts the same method as Example 1 to carry out hygroscopicity t...
Embodiment 3
[0061] Take 32.0mg of epalrestat and 15.4mg of metformin (the molar ratio is 1:1.19) into a 4mL sample bottle, add 2mL of acetonitrile and ethyl acetate, and ultrasonically dissolve it and make it supersaturated. After 48 hours of crystallization, the suspension was centrifuged, the supernatant was discarded, and the centrifuged solid was dried at a room temperature of 25° C. for 12 hours to obtain the epalrestat-metformin salt.
[0062] Carry out XRD test to the product that embodiment 3 obtains, test product is 0.87,11.34,12.78,12.90,15.00,16.29,16.37,16.67,17.48,19.27,19.64,20.28,20.99,21.81,22.87,24.00, There are characteristic peaks at 24.50, 25.05, 26.10, and 26.88. , so the product is the epalrestat-metformin salt.
[0063] The product obtained in Example 3 was tested by DSC. From the test results, there is a sharp endothermic peak at 215.9° C., which is the melting point of epalrestat-metformin salt.
[0064] Embodiment 3 adopts the same method as Example 1 to carry ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com