Eutectic crystal of lenvatinib and benzoic acid, and preparation method thereof
A technology of lenvatinib and benzoic acid, applied in the field of medicinal chemistry, can solve the problems of limited clinical application, poor water solubility of lenvatinib mesylate, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
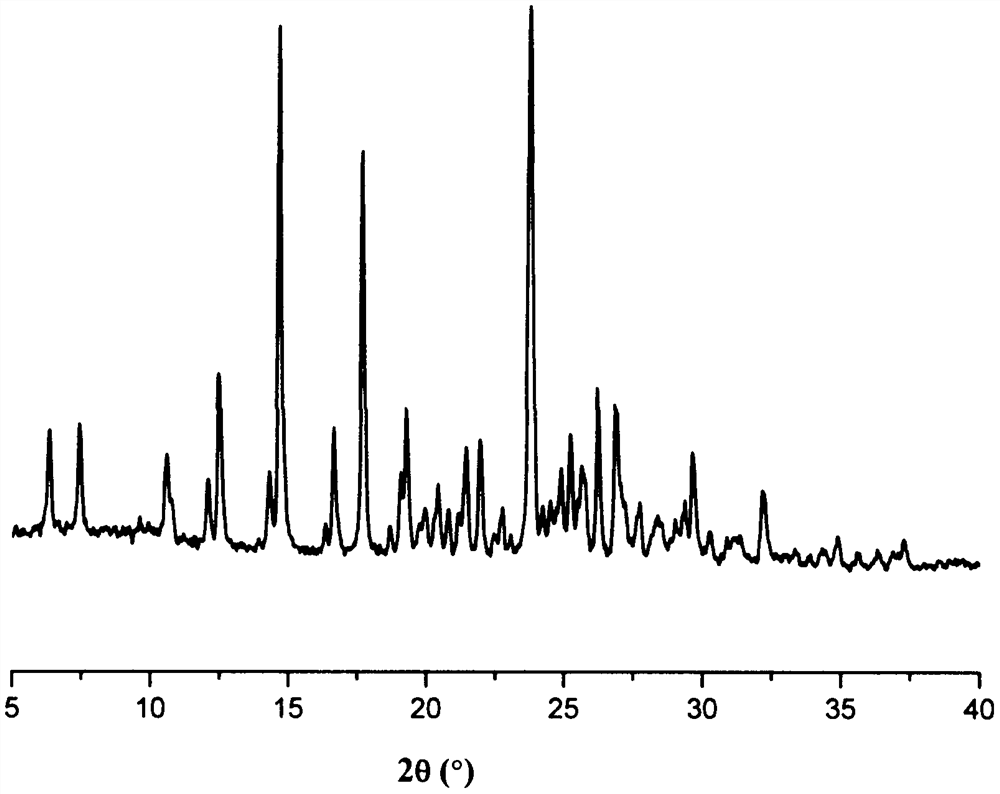
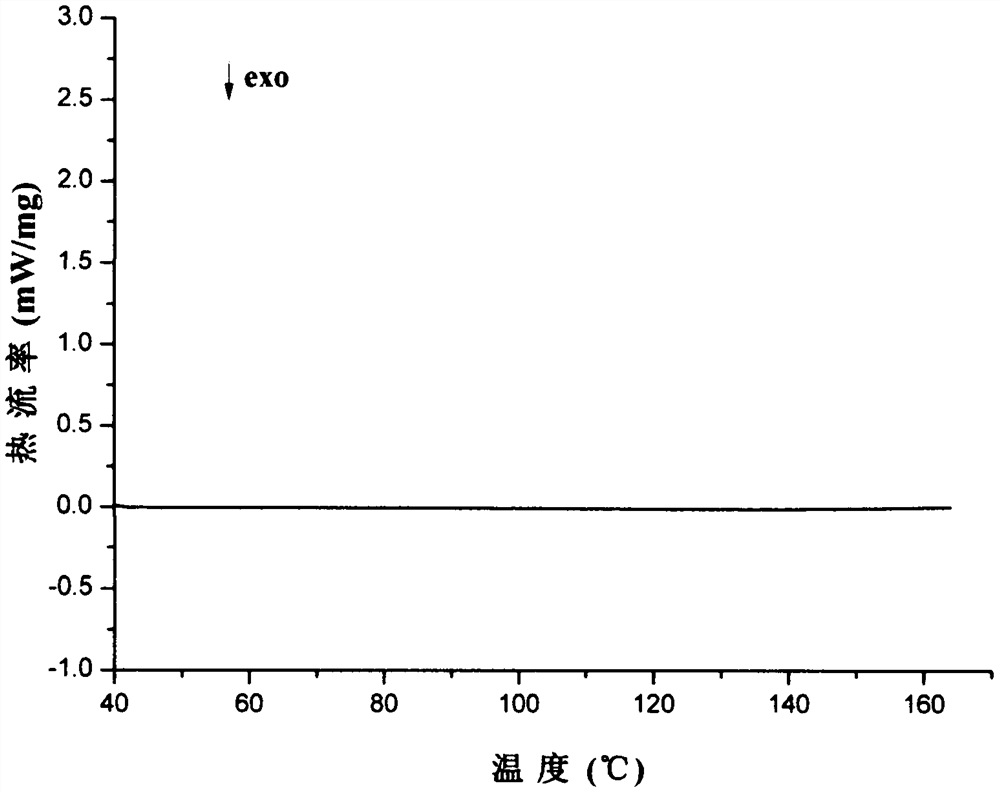
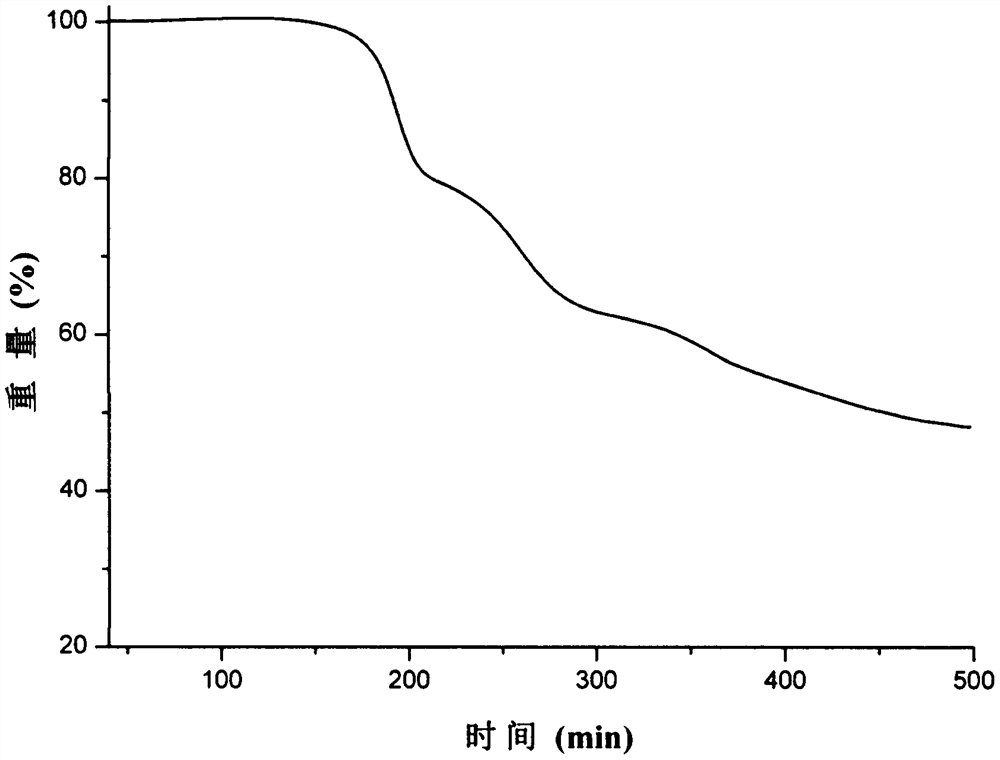
Image
Examples
Embodiment 1
[0040] Weigh 600 mg of lenvatinib and 172 mg of benzoic acid, add 4 mL of acetonitrile and 4 mL of water to obtain a suspension, stir the suspension at room temperature for 1 h, filter, and dry the resulting white solid at 50 ° C to obtain lenvatinib and Solid sample of benzoic acid co-crystal, yield 88.2%.
Embodiment 2
[0042] Weigh 60 mg of lenvatinib and 17.2 mg of benzoic acid, add 1 mL of anisole to obtain a suspension, stir the suspension at room temperature for 12 hours, filter, and dry the obtained white solid at 50°C to obtain lenvatinib Solid sample co-crystallized with benzoic acid.
Embodiment 3
[0044] Weigh 60 mg of lenvatinib and 17.2 mg of benzoic acid, add 10 μL of isopropanol, grind for 30 min at a frequency of 20 Hz, and dry the resulting white solid at 40 ° C to obtain a solid sample of lenvatinib and benzoic acid co-crystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com