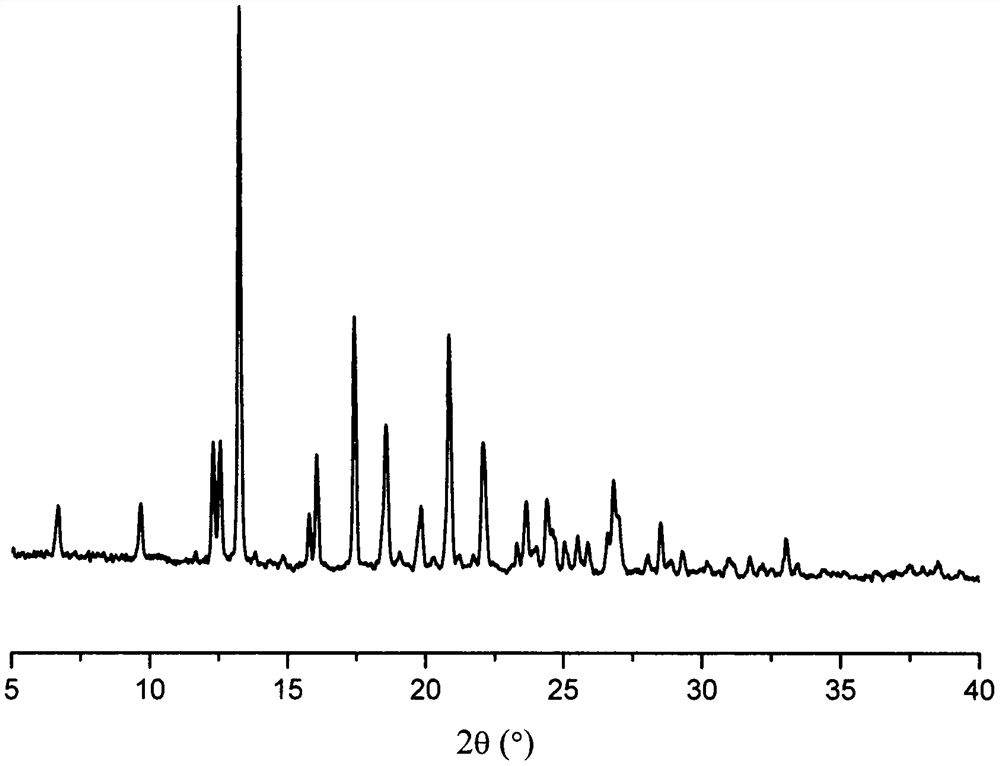
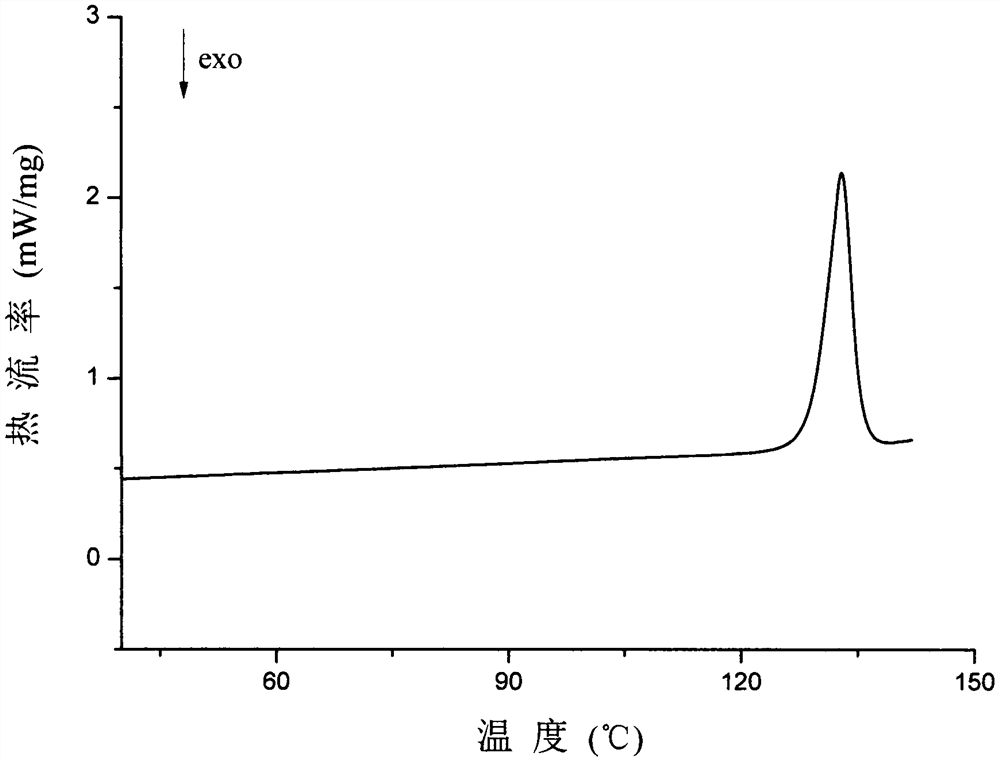
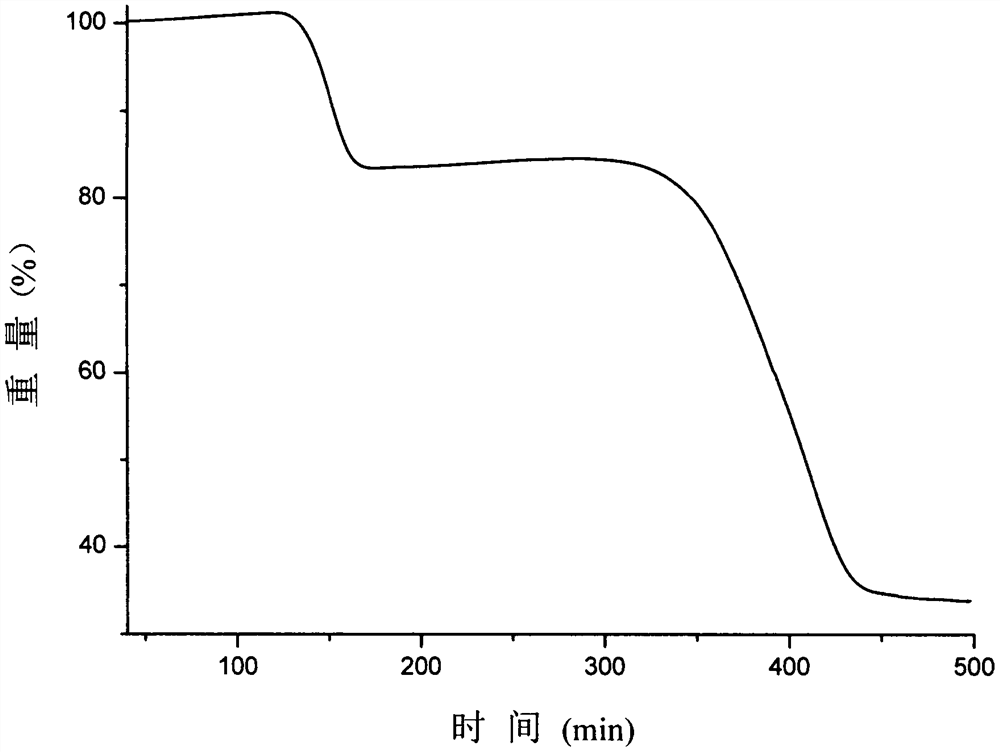
Olaparib and malonic acid eutectic crystal and preparation method thereof
A malonic acid and solvent technology, applied in the field of medicinal chemistry, can solve the problems such as failure to obtain the co-crystal form A of olaparib and urea, low solubility, and limited drug oral absorption efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Weigh 900 mg of olaparib and 216 mg of malonic acid, add 15 mL of n-heptane and 250 μL of ethanol to obtain a suspension, stir the suspension at room temperature for 3 h, filter, and dry the obtained white solid at 40 ° C to obtain A solid sample of the cocrystal of lapani and malonic acid in 90.5% yield.
Embodiment 2
[0040] Weigh 60 mg of olaparib and 14.4 mg of malonic acid, add 1 mL of anisole and 10 μL of ethanol to obtain a suspension, stir the suspension at room temperature for 12 h, filter, and dry the obtained white solid at 40 ° C to obtain A solid sample of the cocrystal of olaparib and malonate.
Embodiment 3
[0042] Weigh 60 mg of olaparib and 14.4 mg of malonic acid, add them to a ball mill jar, then add 10 μL of ethyl acetate, grind at a frequency of 20 Hz for 30 min, and dry the resulting white solid at 40 ° C to obtain olaparib and malonic acid Eutectic solid samples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com