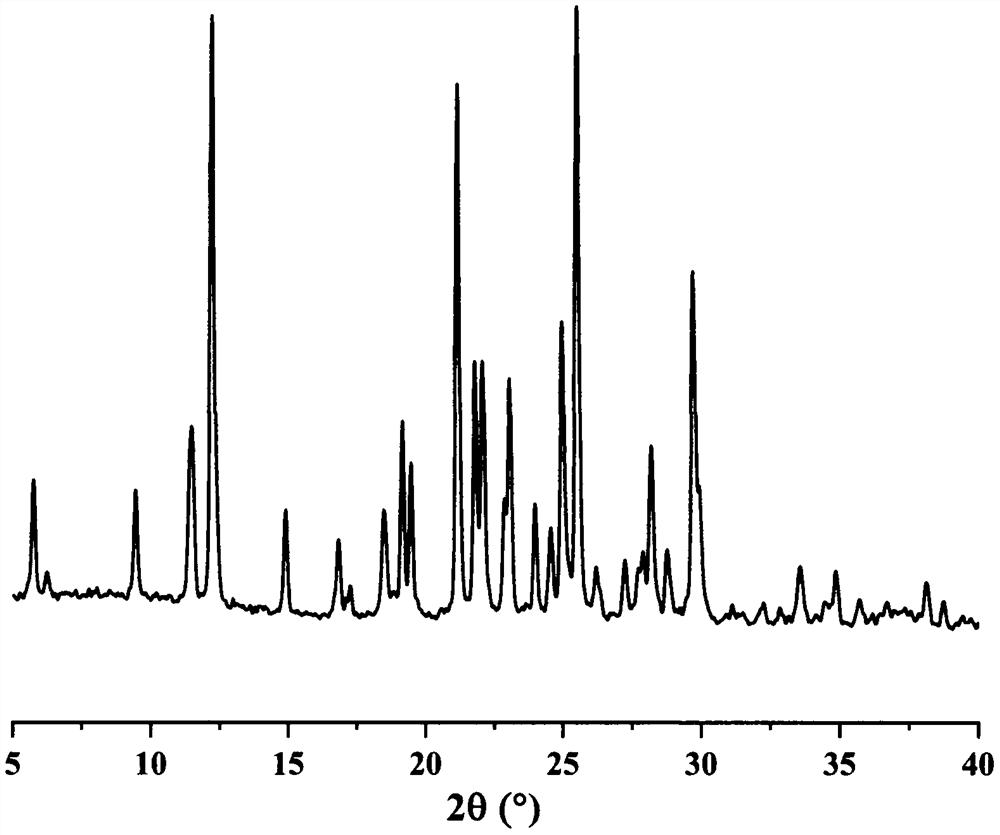
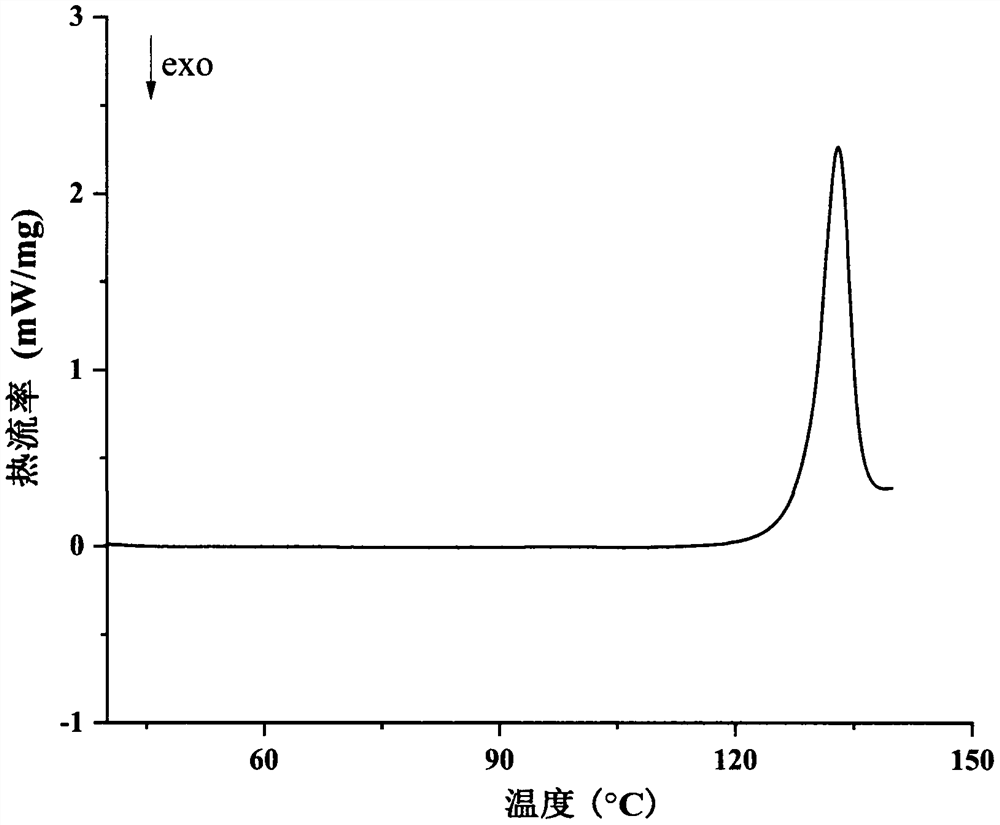
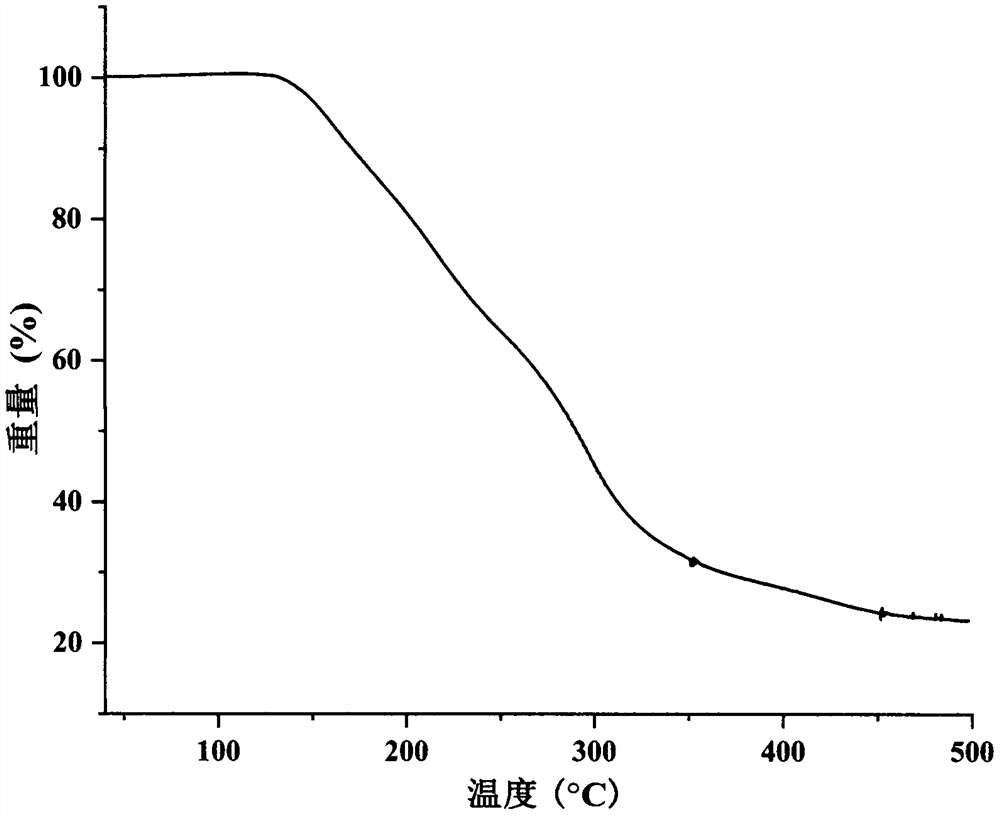
Eutectic crystal of regorafenib and maleic acid and preparation method thereof
A technology of regorafenib and maleic acid, applied in the field of medicinal chemistry, can solve the problems of poor water solubility of regorafenib monohydrate, high price of regorafenib, and limitations in clinical application, and achieve good reproducibility, The effect of easy control of the crystallization process and high apparent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Weigh 1000 mg regorafenib and 240 mg maleic acid, add 20 mL n-heptane and 80 μL methanol to obtain a suspension, stir the suspension at room temperature for 4 h, filter, and dry the obtained white solid at 40 ° C to obtain Rui A solid sample of the cocrystal of gorfenib and maleic acid with a yield of 90.7%.
Embodiment 2
[0037] Weigh 60 mg of regorafenib and 14.4 mg of maleic acid, add 2 mL of n-heptane and 10 μL of ethanol to obtain a suspension, stir the suspension at room temperature for 24 hours, filter, and dry the obtained white solid at 40 ° C to obtain A solid sample of the cocrystal of regorafenib and maleic acid.
Embodiment 3
[0039] Weigh 60mg of regorafenib and 14.4mg of maleic acid, add 2mL of dichloromethane to obtain a suspension, stir the suspension at room temperature for 48h, filter, and dry the obtained white solid at 40°C to obtain regorafenib Solid sample of Ni and maleic acid cocrystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com