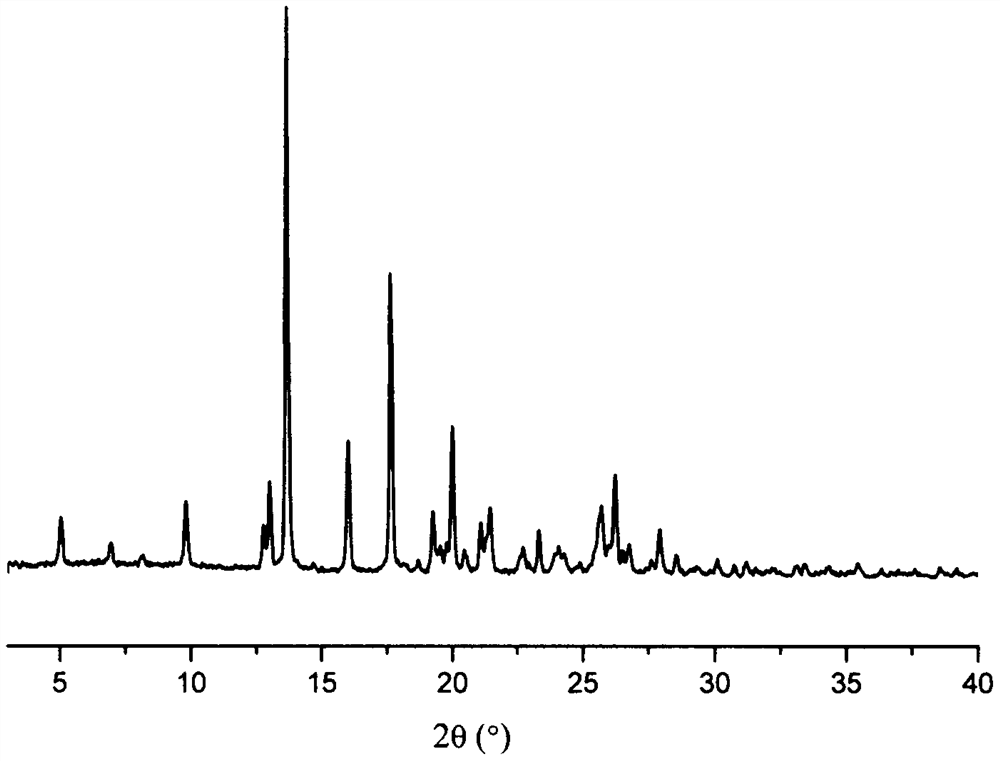
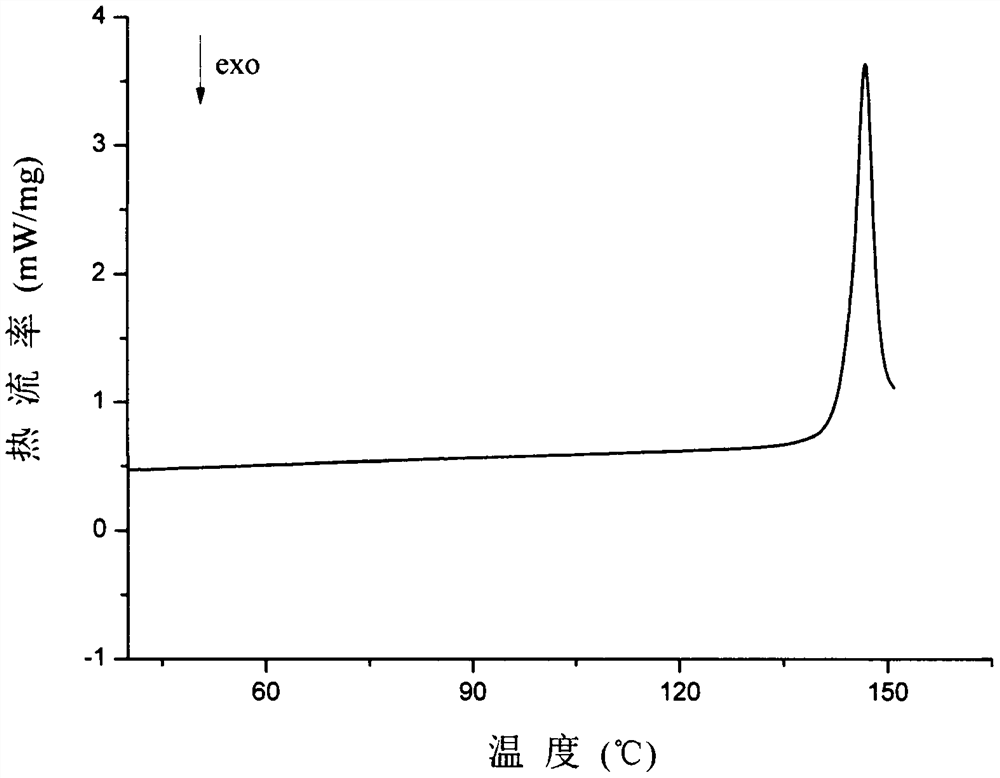
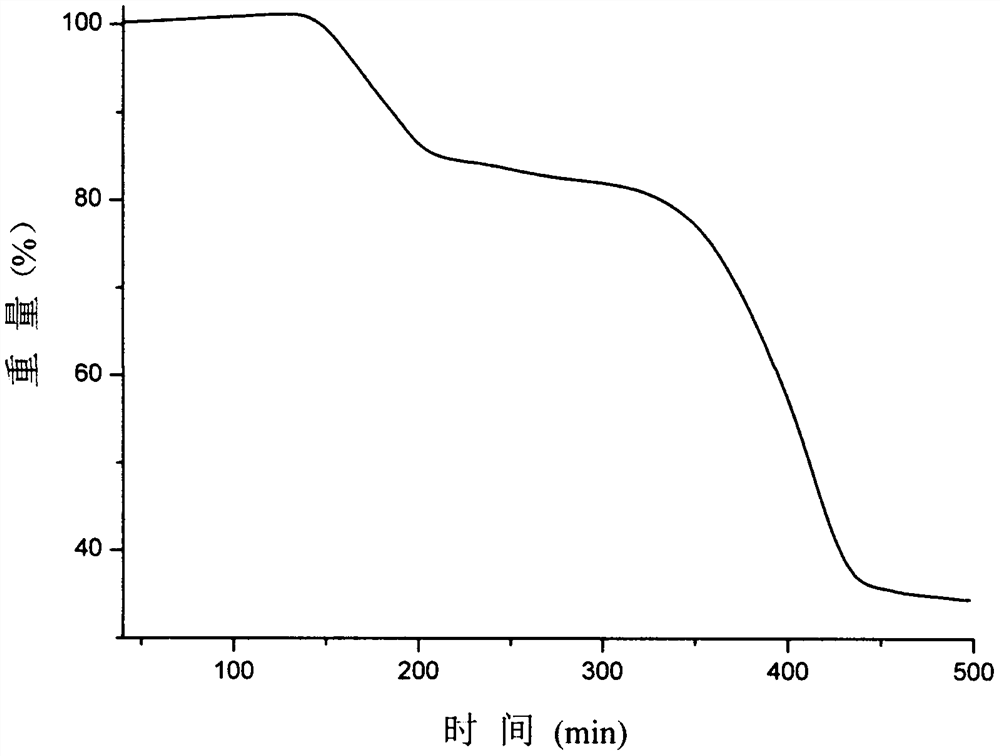
A kind of eutectic of olaparib and maleic acid and preparation method thereof
A technology of maleic acid and solvent, which is applied in the field of medicinal chemistry, can solve the problems of low solubility, limiting the oral absorption efficiency of drugs, failure to obtain the co-crystal form A of olaparib and urea, etc., and achieves a simple preparation method , The crystallization process is easy to control and has good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Weigh 900 mg of olaparib and 240 mg of maleic acid, add 15 mL of n-heptane and 250 μL of ethanol to obtain a suspension, stir the suspension at room temperature for 3 h, filter, and dry the obtained white solid at 40 ° C to obtain Olaparib A solid sample of the co-crystal of lapani and maleic acid with a yield of 93.0%.
Embodiment 2
[0040] Weigh 60 mg of olaparib and 16 mg of maleic acid, add 1 mL of anisole and 10 μL of ethanol to obtain a suspension, stir the suspension at room temperature for 12 h, filter, and dry the obtained white solid at 40 ° C to obtain Olaparib Solid sample of the co-crystal of lapani and maleic acid.
Embodiment 3
[0042] Weigh 60 mg of olaparib and 16 mg of maleic acid, add them to a ball mill jar, then add 10 μL of ethanol, grind at a frequency of 20 Hz for 30 min, and dry the resulting white solid at 40 ° C to obtain a cocrystal of olaparib and maleic acid. solid sample.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com