Metformin-tolbutamide new salt type and preparation method and medical application thereof
A technology of tolbutamide and metformin, which is applied in the field of medicine and chemical industry, can solve the problems of metformin instability, poor pharmaceutical properties, low solubility, etc., and achieve easy control of the crystallization process, improved hygroscopicity and stability, and improved solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
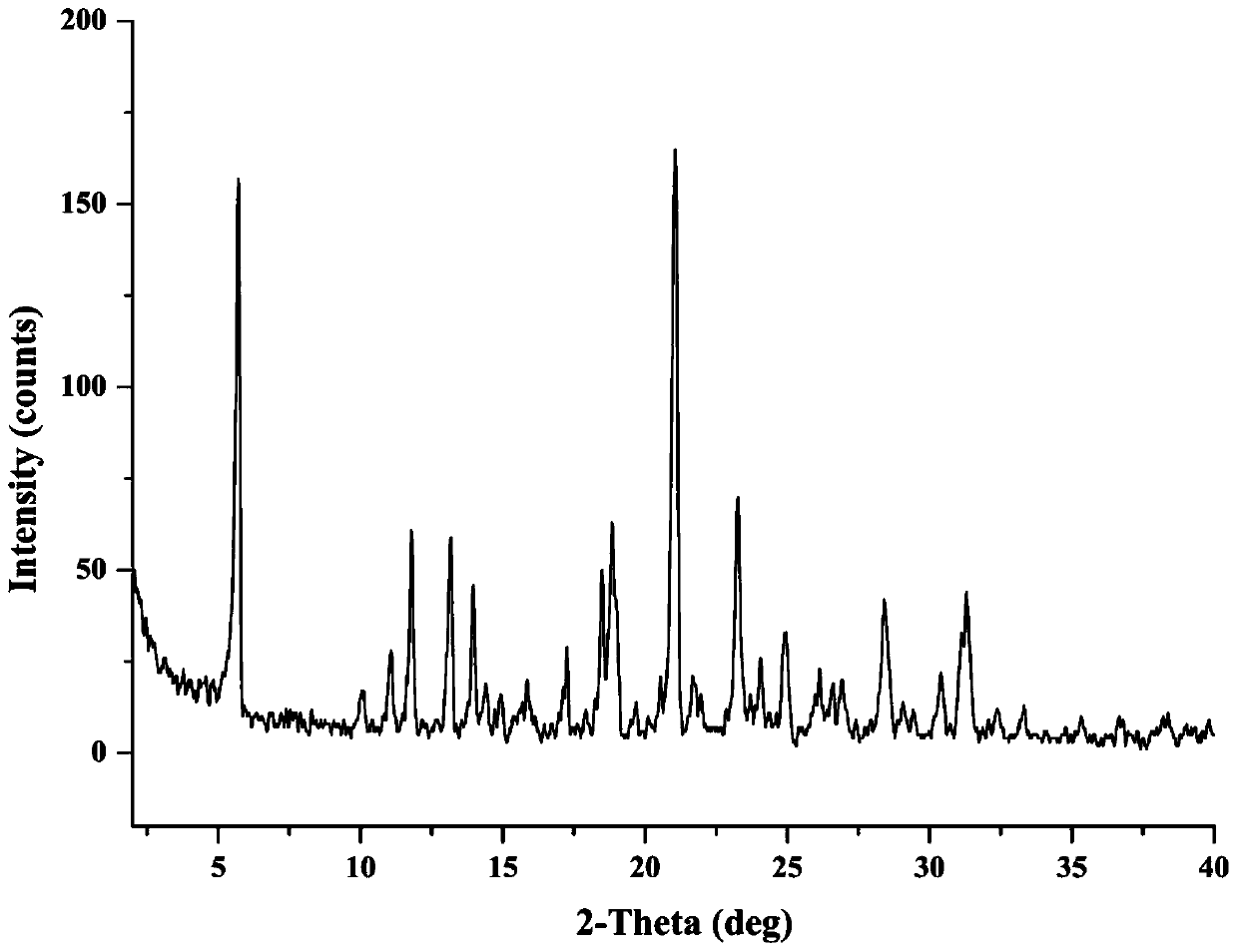
[0041] Take 21.6mg of tolbutamide and 10.8mg of metformin in a 4mL sample bottle, add 2mL of ethyl acetate, ultrasonically dissolve and become supersaturated, react and crystallize at room temperature for 24 hours, centrifuge the suspension, discard Remove the supernatant, and dry the centrifuged solid in a blast oven at 40°C for 3 hours to obtain the metformin-tolbutamide salt form. The XRPD results are as follows: figure 1 .
Embodiment 2
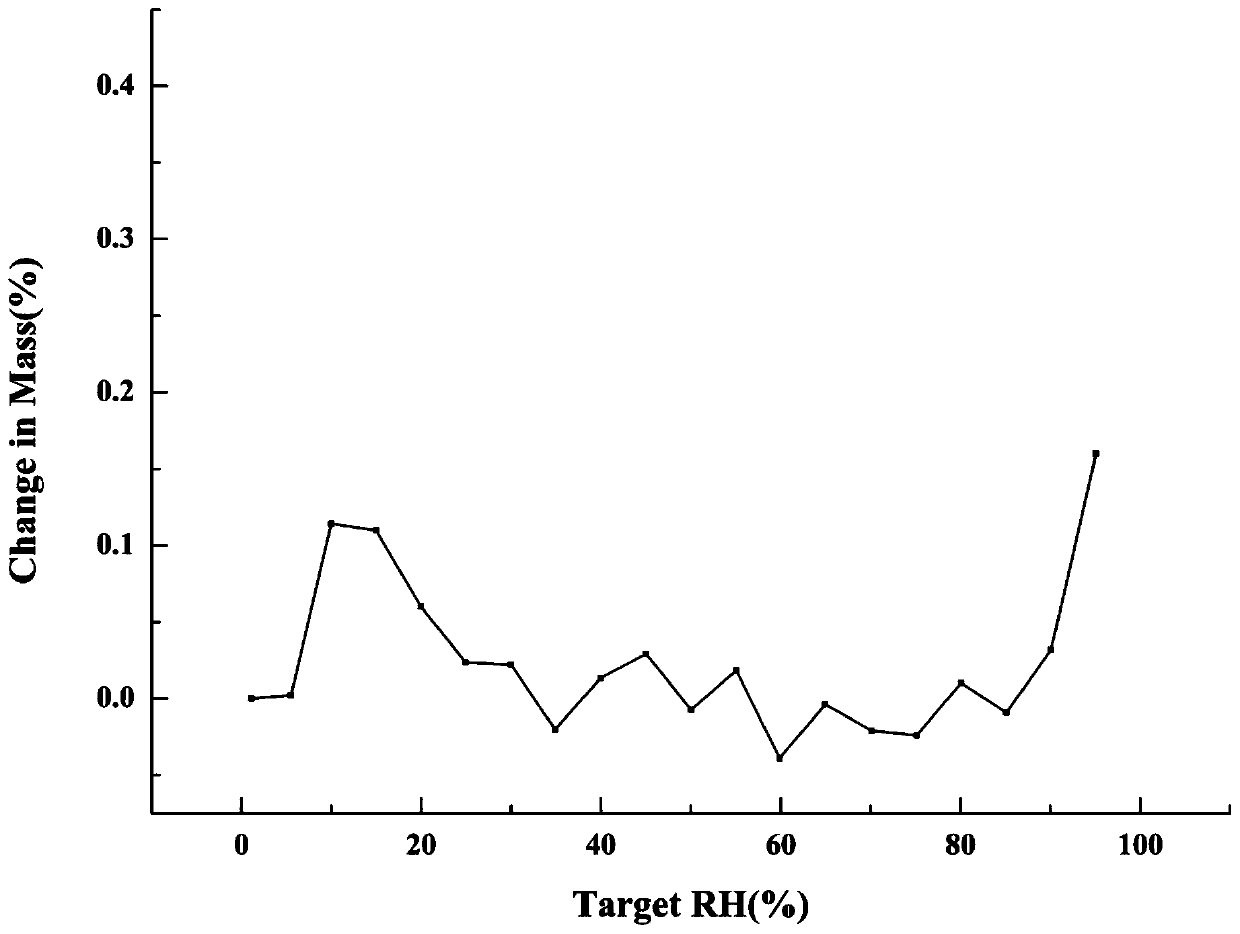
[0043] Get about 3 mg of the metformin-tolbutamide salt type solid sample prepared in Example 1 and carry out dynamic moisture adsorption analysis, using VTI-SA + Determination by dynamic moisture adsorption instrument. The temperature is 25°C and the relative humidity range is 1-95%. The result shows that the water content of metformin-tolbutamide salt type changes little with the change of relative humidity, and when from 1% relative humidity to 95% relative humidity, the mass percentage of its water content does not have obvious increase, as specification attached image 3 , and the salt type remains unchanged, the XRPD pattern is as follows Figure 5 . And the known metformin prototype medicine draws wet weight gain obviously under this relative humidity condition, has been up to 86%. Figure 4 .
Embodiment 3
[0045] Take 21.6mg of tolbutamide and 10.8mg of metformin in a 4mL sample bottle, add 2mL of acetone, ultrasonically dissolve and become supersaturated, react and crystallize at room temperature for 24 hours, centrifuge the suspension, discard the upper Clear liquid, dry the centrifuged solid in a blast oven at 40°C for 3 hours to obtain the metformin-tolbutamide salt form, and the XRPD results are as follows: Figure 9 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com