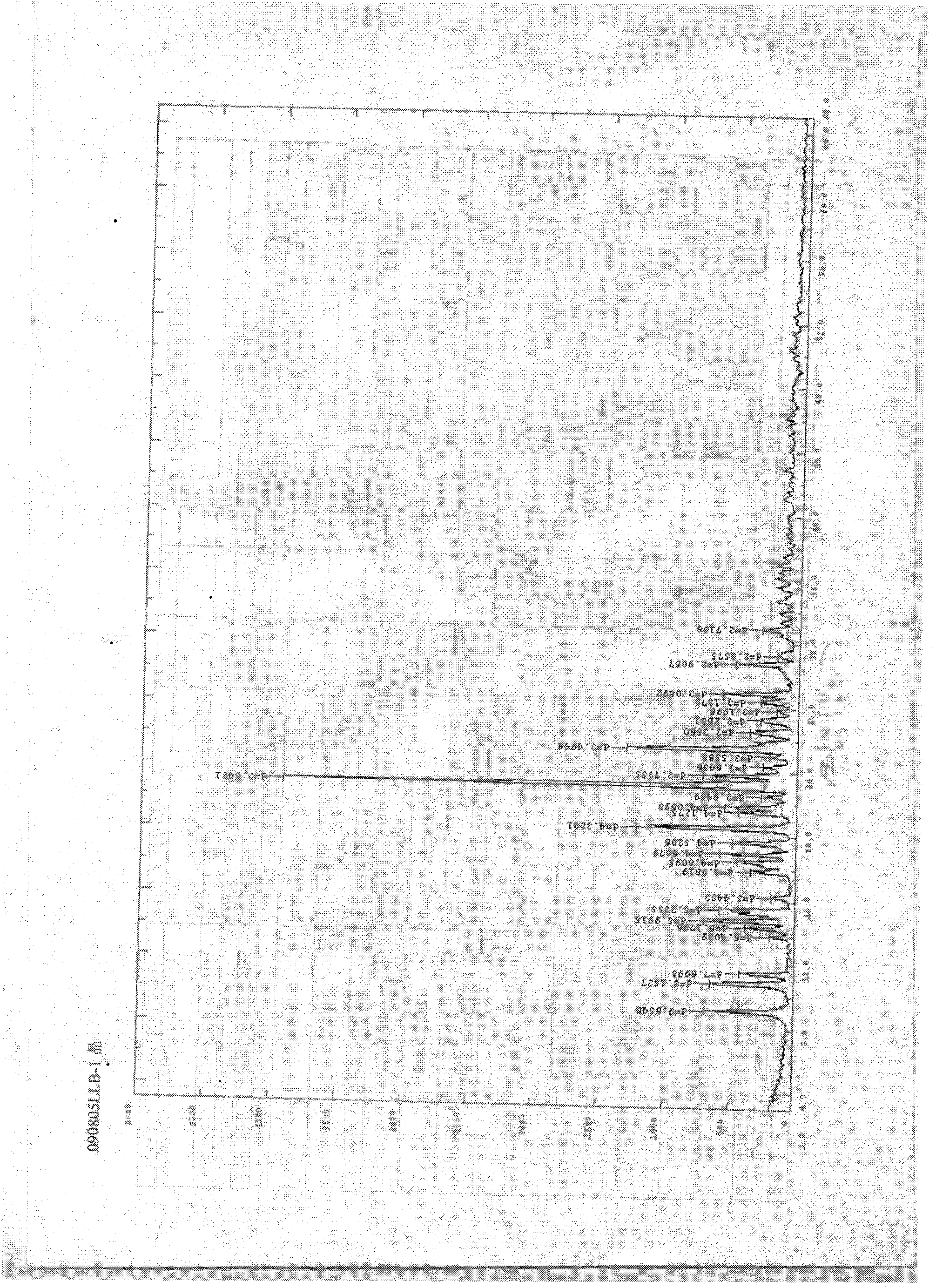
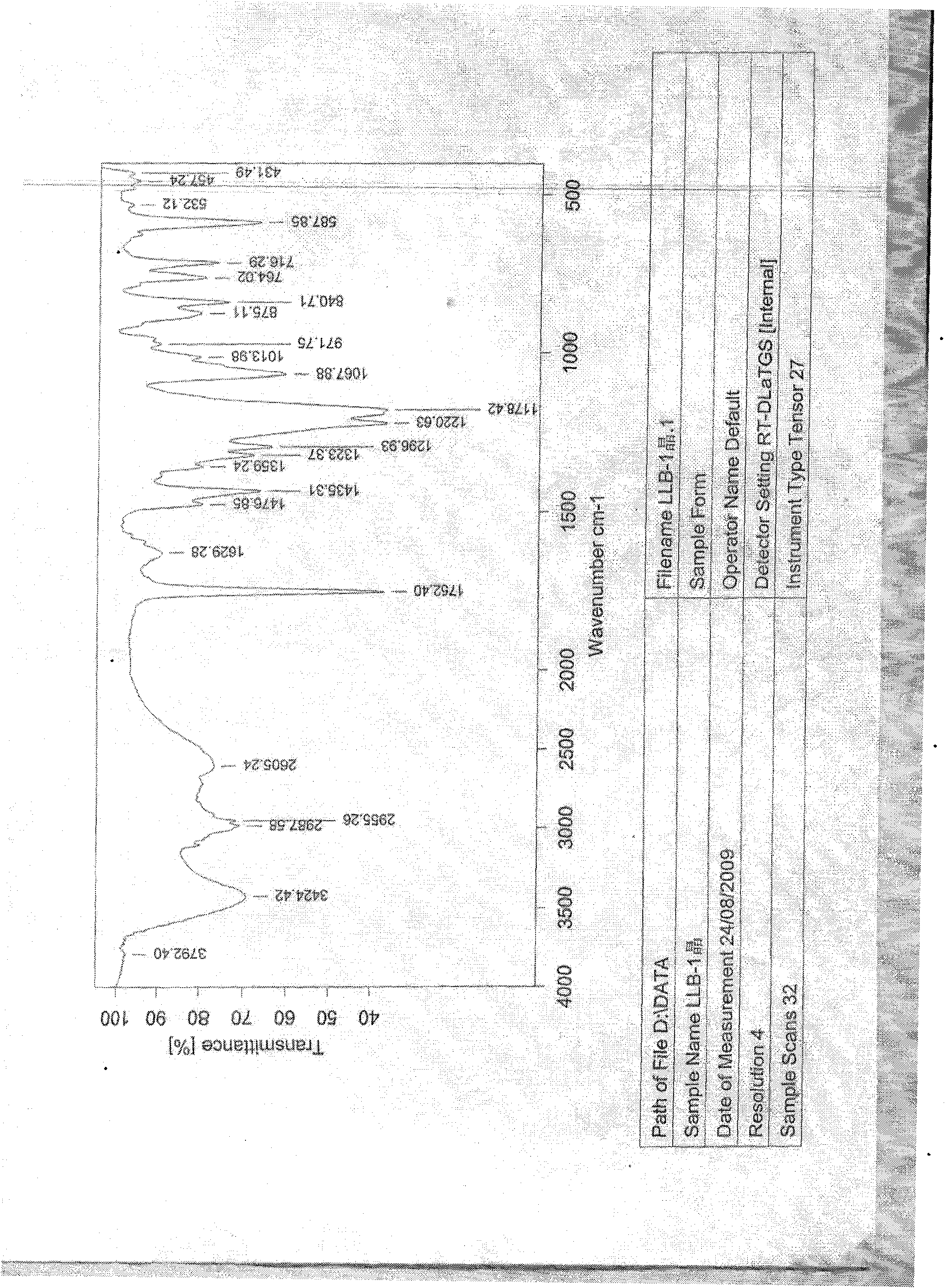
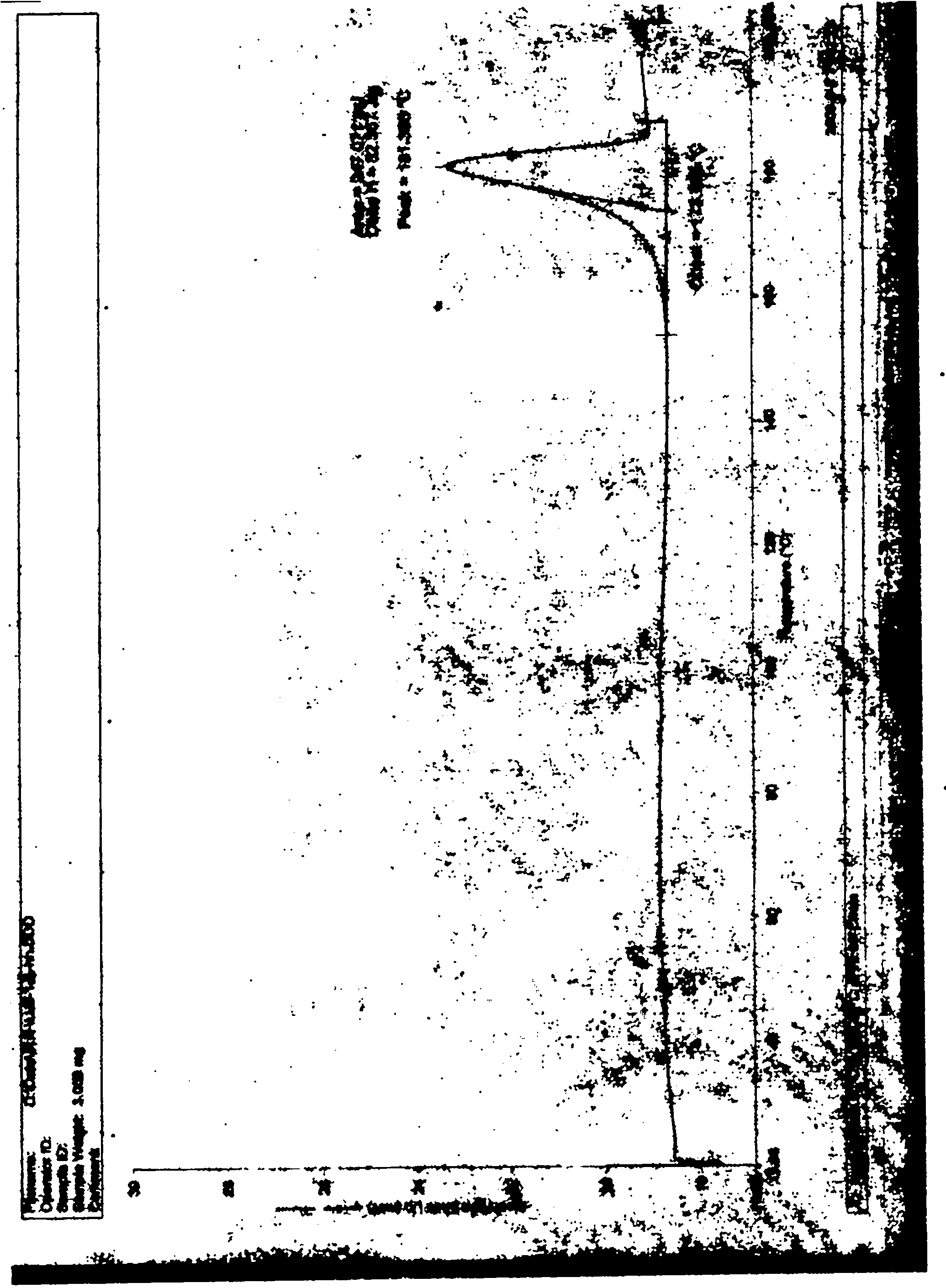
Crystallizing method for obtaining I crystal form (+)-(S)-clopidogrel bisulfate
A technology of clopidogrel bisulfate and clopidogrel free base, which is applied in the field of antithrombotic drugs, can solve the problems of no obvious improvement in the crystallization process, difficult to precipitate solids, and harsh conditions, and achieve simple and easy-to-control crystallization process with high yield , good crystal fluidity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In a 100ml three-necked flask, add (+)-(S)-clopidogrel free base 5.00g, propanol 25ml, stir to dissolve, ice bath controls temperature and slowly adds dropwise 1.5g of concentrated sulfuric acid (mass concentration is 98%) 25ml of propanol solution, after the dropwise addition, was incubated and stirred for 10h to obtain a large amount of white solid, which was filtered and dried under reduced pressure to constant weight to obtain 4.75g I crystal form (+)-(S)-clopidogrel hydrogensulfate. The rate is 73%.
Embodiment 2
[0019] In a 100ml three-necked flask, add (+)-(S)-clopidogrel free base 5.00g, butanol 25ml, stir and dissolve, and slowly add 1.5g of concentrated sulfuric acid (mass concentration is 98%) dropwise to control the temperature in an ice bath. 25ml of butanol solution, after the dropwise addition, was incubated and stirred for 10h to obtain a large amount of white solid, which was filtered and dried under reduced pressure to constant weight to obtain 5.01g I crystal form (+)-(S)-clopidogrel hydrogensulfate. rate of 77%.
Embodiment 3
[0021] In a 100ml three-necked flask, add (+)-(S)-clopidogrel free base 5.00g, butanol 100ml, stir to dissolve, control the temperature in an ice bath and slowly add 1.5g of concentrated sulfuric acid (mass concentration is 98%) dropwise 100ml of butanol solution, after the dropwise addition, was incubated and stirred for 12h to obtain a white solid, filtered, and dried under reduced pressure to constant weight to obtain 2.73g I crystal form (+)-(S)-clopidogrel hydrogensulfate, yield 41.96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com