Icariin form-alpha crystal, preparation method thereof and medicinal combination and application thereof
A technology of icariin and crystal form, applied in the field of medicinal chemistry, can solve the problems of poor water solubility of icariin, limited clinical application, poor oral absorption, etc., achieves good reproducibility, high oral absorption rate, and is conducive to absorption and the effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Take 10 mg of icariin and heat it to 180°C for 5 minutes to obtain the Formα crystal form of icariin.
Embodiment 2
[0037] Take 30 mg of icariin and heat it to 180°C for 10 minutes to obtain the Formα crystal form of icariin.
Embodiment 3
[0039] Take 50 mg of icariin and heat it to 180°C for 15 minutes to obtain the Formα crystal form of icariin.
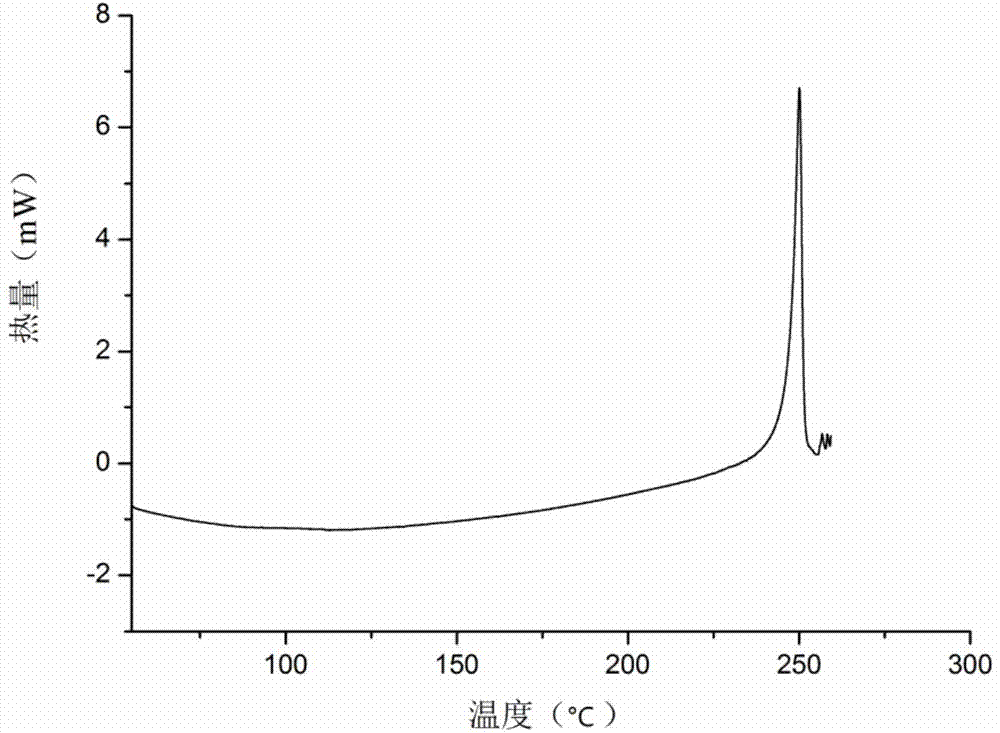
[0040] A kind of icariin Formα crystal form provided by the present invention, through X-ray powder diffraction (XRPD), thermogravimetric analysis (TG), differential scanning calorimetry (DSC), dynamic water adsorption (DVS), infrared ( IR) and Raman (Raman) and other solid-state methods for characterization.
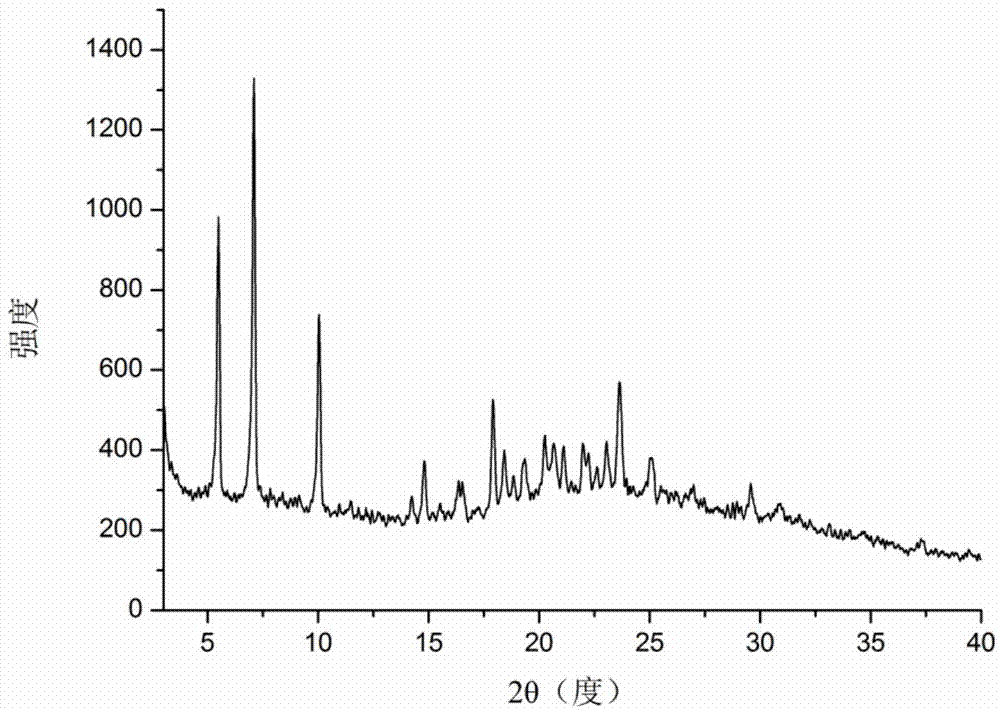
[0041] Carry out X-ray powder diffraction analysis to the icariin Form α crystal form solid sample that embodiment 1 makes, it adopts the diffractometer of Bruker D8advance type of German Bruker Instrument Co., Ltd., adopts Cu-K α ray The voltage is 40 kV, the current is 40 mA, the scanning speed is 12 degrees per minute, the step size is 0.02 degrees, and each step takes 0.1 seconds. The results of the analysis can be found in figure 1 .
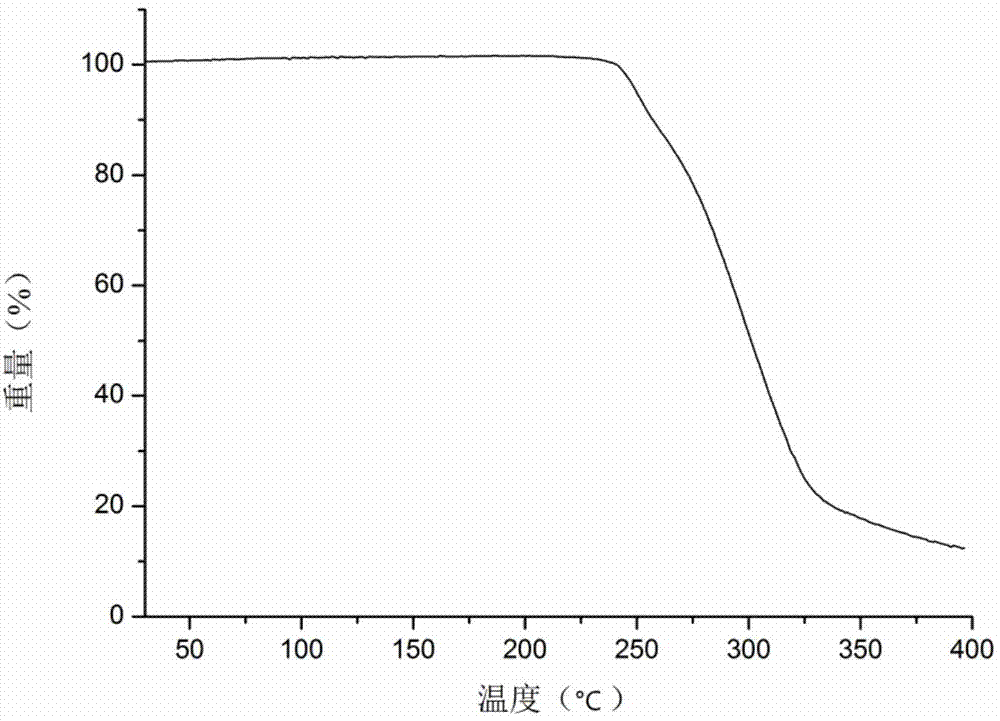
[0042] The icariin Formα crystal solid sample prepared in Example 1 was subjected to thermogravimetric analysis using...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com