Cyclovirobuxine D sublingual tablet as well as preparation method and application thereof
A technology of cyclovir buxus and sublingual tablets, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, pill delivery, etc., can solve problems such as unfavorable industrial production, complex types of auxiliary materials, and cumbersome adding procedures, etc. The effect of less, low production cost and high drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 Preparation of sublingual tablet of the present invention
[0026] 【prescription】
[0027]
[0028] 【Preparation】
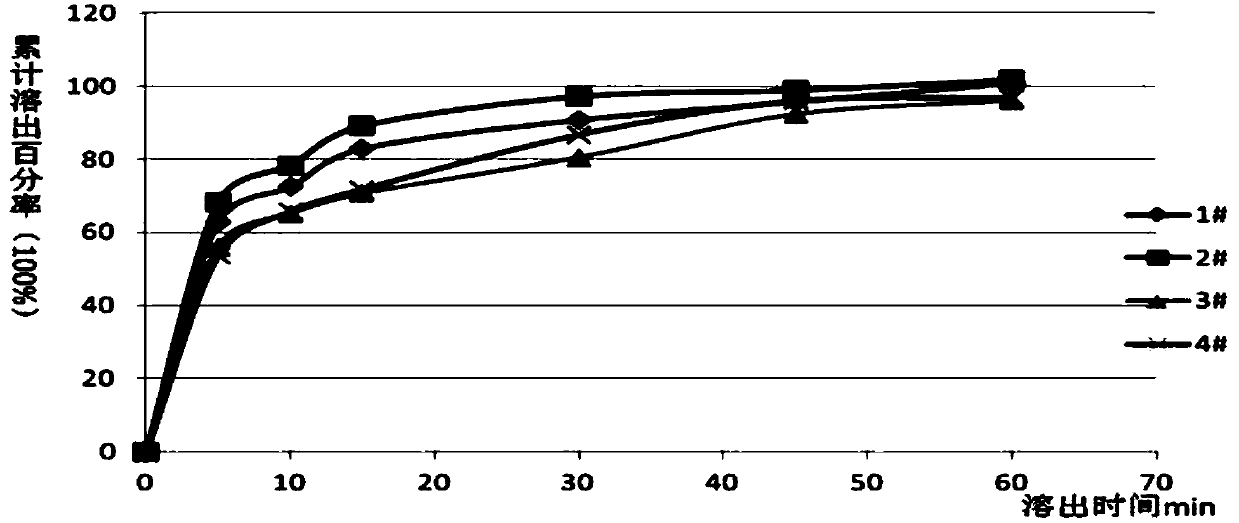
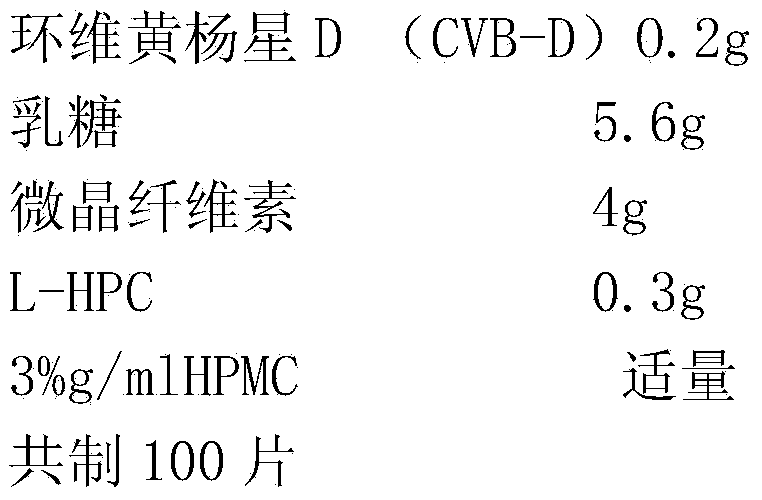
[0029] First, mix the drug CVB-D with 2 / 3 of the prescription amount of the disintegrant low-substituted hydroxypropyl cellulose (L-HPC), and then add the filler lactose and microcrystalline cellulose in equal increments, and mix well. Add an appropriate amount of binder (10% starch slurry) to make the prepared soft material "lightly hold into a ball and disperse when pressed", pass through a 20-mesh sieve, and dry the obtained wet granules. After sizing, add the remaining 1 / 3 of the disintegrant, ready to be compressed into tablets. Embodiment 2 Preparation of sublingual tablet of the present invention
Embodiment 2
[0029] First, mix the drug CVB-D with 2 / 3 of the prescription amount of the disintegrant low-substituted hydroxypropyl cellulose (L-HPC), and then add the filler lactose and microcrystalline cellulose in equal increments, and mix well. Add an appropriate amount of binder (10% starch slurry) to make the prepared soft material "lightly hold into a ball and disperse when pressed", pass through a 20-mesh sieve, and dry the obtained wet granules. After sizing, add the remaining 1 / 3 of the disintegrant, ready to be compressed into tablets. Embodiment 2 Preparation of sublingual tablet of the present invention
[0030] 【prescription】
[0031]
[0032] 【Preparation】
[0033] First, mix the drug CVB-D with 2 / 3 of the prescription amount of the disintegrant low-substituted hydroxypropyl cellulose (L-HPC), and then add the filler lactose and microcrystalline cellulose in equal increments, and mix well. Add an appropriate amount of binder (10% starch slurry) to make the prepared so...
Embodiment 3
[0033] First, mix the drug CVB-D with 2 / 3 of the prescription amount of the disintegrant low-substituted hydroxypropyl cellulose (L-HPC), and then add the filler lactose and microcrystalline cellulose in equal increments, and mix well. Add an appropriate amount of binder (10% starch slurry) to make the prepared soft material "lightly hold into a ball and disperse when pressed", pass through a 20-mesh sieve, and dry the obtained wet granules. After sizing, add the remaining 1 / 3 of the disintegrant, ready to be compressed into tablets. Embodiment 3 Preparation of sublingual tablet of the present invention
[0034] 【prescription】
[0035]
[0036]
[0037] 【Preparation】
[0038] First, mix the drug CVB-D with 2 / 3 of the prescription amount of the disintegrant low-substituted hydroxypropyl cellulose (L-HPC), and then add the filler lactose and microcrystalline cellulose in equal increments, and mix well. Add an appropriate amount of binder (10% starch slurry) to make the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com