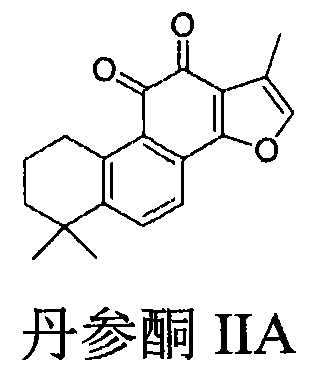
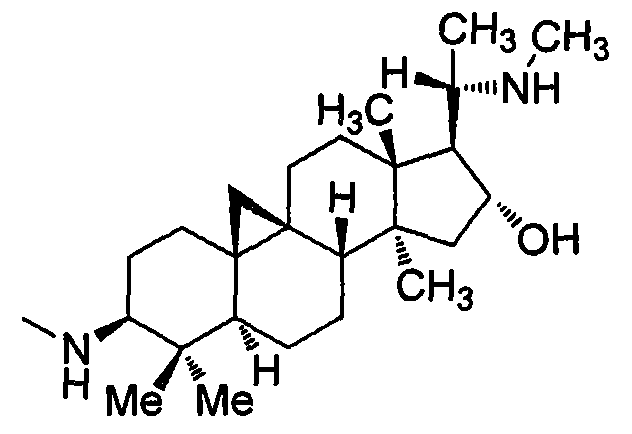
Preparation of compound with tanshinone IIA and cyclovirobuxine D composite structure and application of compound in prevention of cardiovascular diseases
A technology of cyclovirboxicin and composite structure, which is applied to the preparation of a class of compounds with a composite structure of tanshinone IIA and cyclovirboxicin D and its application in the prevention of cardiovascular diseases, and can solve the problem of poor water solubility and cyclovirboxicin Star D has problems such as narrow use range, to achieve the effect of maintaining activity, eliminating potential risks, and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of 9β,19-cyclo-16α-hydroxy-4,4,13,14-tetramethyl-3-methylamino-17-[1-(tanshinone IIA)amino]ethylpregnane (A)
[0043]
[0044] Step 1: Synthesis of Bromotanshinone IIA Compound A-1
[0045] Add compound tanshinone IIA (1.00g, 3.4mmol) and 80 ml of acetic acid in the flask, add Br dropwise therein 2(0.54g, 3.4mmol) in 20ml of acetic acid solution, stirred at room temperature for 4 hours. Dilute with water, extract with DCM, combine the extracts, wash with water, sodium sulfite aqueous solution, saturated sodium chloride, and dry over anhydrous magnesium sulfate. Column chromatography (petroleum ether / ethyl acetate=30 / 1) gave red solid A-1 (1.012 g, yield 80%)
[0046] 1 H NMR (300MHz, CDCl 3 )δ7.63(d, 1H), 7.52(d, 1H), 3.16(t, 2H), 2.2(s, 3H), 1.78(m, 2H), 1.65(m, 2H), 1.3(s, 6H )
[0047] EIMS (m / z): 374, 372
[0048] Step 2: 9β,19-cyclo-16α-hydroxy-4,4,13,14-tetramethyl-3-methylamino-17-[1-(tanshinone IIA group)amino]ethylpregnane (A ) preparatio...
Embodiment 2
[0054] 9β,19-Cyclo-16α-Hydroxy-4,4,13,14-Tetramethyl-3-methylamino-17-[1-(tanshinone IIA methylene)amino]ethylpregnane (B ) preparation
[0055]
[0056] Mix 1.56 g of tanshinone IIA, 0.5 ml of 37% formaldehyde solution, 7.4 g of cyclovirbuxine D, and 50 ml of acetic acid, heat and reflux in an oil bath for 10 hours, remove the solvent from the mixture under reduced pressure to obtain a red solid, which is dissolved in 30 ml of DCM, Add 10 ml of saturated sodium carbonate for washing, separate the layers, dry the filtrate, concentrate, and separate by column chromatography on silica gel (eluent: DCM / methanol = 10:1) to obtain 1.12 g (yield 36%) of compound B.
[0057] 1 H-NMR (CDCl 3 )δ7.34(d, 1H), 7.25(d, 1H), 3.48(s, 2H), 3.22-3.26(m, 5H), 2.9(t, 2H), 2.8(m, 1H), 2.6(m , 1H), 2.3(s, 3H), 1.95(s, 3H), 1.2-1.8(m, 19H), 1.04(s, 6H), 0.98(s, 6H), 0.89(s, 6H)
[0058] LCMS m / z, 709.5(M+1) +
[0059] Purity: HPLC-UV (280nm): 99.1%.
Embodiment 3
[0061] 9β,19-cyclo-16α-hydroxy-4,4,13,14-tetramethyl-3-methylamino-17-[1-(tanshinone IIA acyl)amino]ethylpregnane (C) preparation
[0062]
[0063] Step 1: Preparation of intermediate C-1 aldehyde tanshinone IIA
[0064] Add 1 mL of dry DMF to a dry flask, and add 0.2 mL of POCl dropwise under stirring 3 , stirred for 15 minutes, and a solution of tanshinone IIA (287 mg, 1.3 mmol) in 3 mL of DMF was added. React overnight at 80-85 degrees. Cool to room temperature, pour into ice water, adjust pH to 7, extract with ethyl acetate, combine organic phases, wash with water, wash with saturated sodium chloride, dry over anhydrous magnesium sulfate, simple column chromatography (petroleum ether / ethyl acetate=20 / 1) The oily liquid C-1 was obtained, which was directly used in the next reaction.
[0065] Step 2: Preparation of Intermediate C-2 Tanshinone IIA Formic Acid
[0066] Add the above obtained liquid into 10 ml of 0.3N potassium permanganate solution and stir overnight ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com