Medicinal composition for treating ischemic cerebrovascular disease, and preparation method thereof
A cerebrovascular disease and composition technology, applied in the field of pharmaceutical preparations containing organic active ingredients, can solve problems such as unsatisfactory effects, and achieve the effects of improving bioavailability, good therapeutic effect, and clear ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Embodiment 1: (nasal cavity spray)
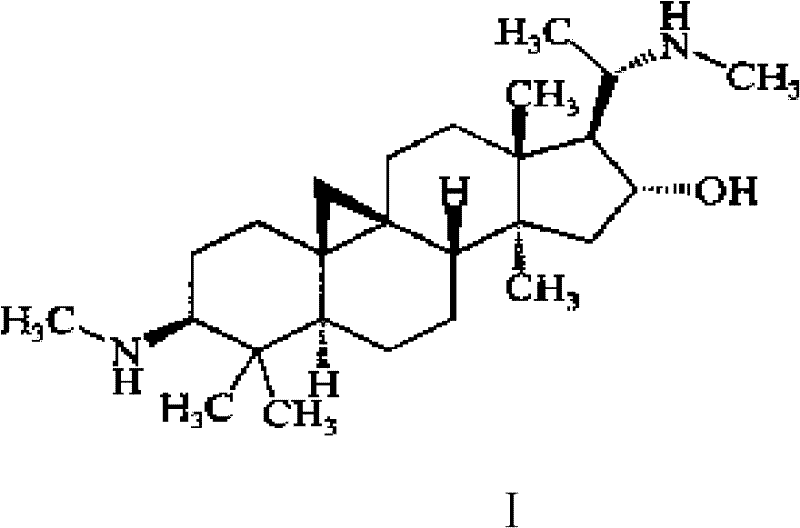
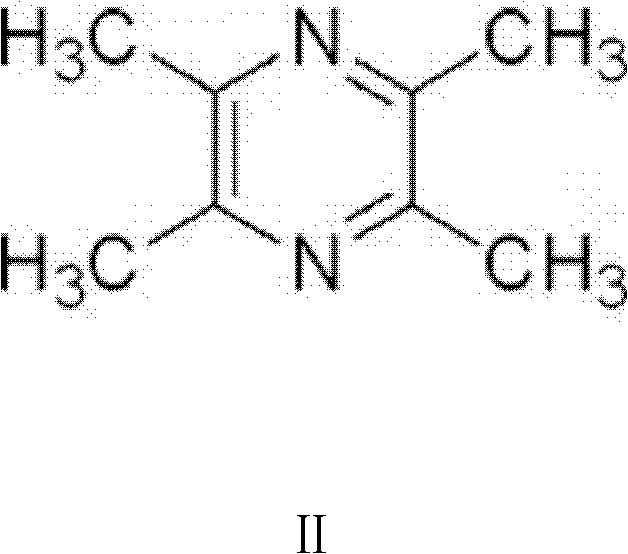
[0104] 1. Active ingredients: 5g of Cycloviral Buxus D, 30g of Ligustrazine, 2g of Borneol.
[0105] 2. Preparation method:
[0106] (1) Take borneol and dissolve it with an appropriate amount of ethanol to obtain a borneol ethanol solution for later use; take 0.15 g of hydroxypropyl-β-cyclodextrin and add 3 times of distilled water to grind it evenly, add the prepared borneol ethanol solution dropwise, grind and clathrate at room temperature After 1.5 hours, the solution was refrigerated for 24 hours, washed 3 times with ethyl acetate, filtered through a G5 vertical melting funnel, and the filtrate was vacuum-dried to obtain borneol HP-β-cyclodextrin inclusion complex;
[0107] (2) Cyclovirbuxine D and Ligustrazine are dissolved in distilled water, then add the borneol HP-beta-cyclodextrin inclusion compound and ethylparaben 0.1g that step (1) makes successively, fully mix Mix well, add triethanolamine to adjust the pH value to 5....
Embodiment 2
[0108] Embodiment 2: (nasal cavity spray)
[0109] 1. Active ingredients: 7.5g Cycloviral Buxusin D, 40g Ligustrazine, 3.5g Borneol.
[0110] 2. Preparation method:
[0111](1) Take borneol and dissolve it with an appropriate amount of ethanol to obtain borneol ethanol solution for later use; take 0.25 g of hydroxypropyl-β-cyclodextrin and add 3 times of distilled water to grind it evenly, add the prepared borneol ethanol solution dropwise, and grind at room temperature for inclusion After 1.5 hours, the solution was refrigerated for 24 hours, washed 3 times with ethyl acetate, filtered through a G5 vertical melting funnel, and the filtrate was vacuum-dried to obtain borneol HP-β-cyclodextrin inclusion complex;
[0112] (2) Dissolve Cyclovirbuxine D and Ligustrazine in distilled water, then add the borneol HP-β-cyclodextrin inclusion complex prepared in step (1), carbomer 5g, hydroxypropyl methyl Mix 14g of cellulose and 0.2g of potassium sorbate, add triethanolamine to adju...
Embodiment 3
[0113] Embodiment 3: (nasal cavity spray)
[0114] 1. Active ingredients: 10g of Cycloviral Buxusin D, 50g of Ligustrazine, and 5g of Borneol.
[0115] 2. Preparation method:
[0116] (1) Take borneol and dissolve it with an appropriate amount of ethanol to obtain a borneol ethanol solution for later use; take 0.375 g of hydroxypropyl-β-cyclodextrin and add 3 times of distilled water to grind it evenly, add the prepared borneol ethanol solution dropwise, grind and clathrate at room temperature After 1.5 hours, the solution was refrigerated for 24 hours, washed 3 times with ethyl acetate, filtered through a G5 vertical melting funnel, and the filtrate was vacuum-dried to obtain borneol HP-β-cyclodextrin inclusion complex;
[0117] (2) Dissolve Cyclovirbuxine D and Ligustrazine in distilled water, and then add the borneol HP-β-cyclodextrin inclusion compound, Poloxamer 188 14g, Poloxa Mix 18g of M407 and 0.5g of ethyl hydroxybenzoate, add triethanolamine to adjust the pH value...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com