Cyclovirobuxine D raw medicine and method for determining its content in preparation by chromatography
The technology of Cyclovitamin and bulk medicine is applied to the detection of evaporative light scattering detector. , In the field of high performance liquid chromatography, it can solve the problems of cumbersome operation, poor specificity, light and humidity sensitivity, etc., and achieve the effect of simple operation method, simple method and simple operation
Inactive Publication Date: 2010-12-01
TIANJIN TASLY PHARMA CO LTD
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The main component of Buxus Ning, Cyclovitamin D, and other components are all alkaloids, so the non-aqueous titration method recorded on page 153 of the 2000 edition of the Chinese Pharmacopoeia is a classic method for determining alkaloids. This method is simple, but the specificity is poor. That is, as long as it is an alkaloid, it can be detected, so the result obtained is the total alkaloid content of the alkaloid, that is, the total amount of cyclovirboxicin D and other components, and cyclovirboxicin D is only one of the alkaloids. This detection method cannot accurately determine the true content of Cyclovitamin D
The preparation Huangyangning tablets produced with Huangyangning as the original drug, the method contained in page 575 of its "Chinese Pharmacopoeia" 2000 edition is the acid dye colorimetric method, which is cumbersome to operate and has poor specificity. can be detected, so the result obtained is the total alkaloid content of alkaloids, that is, the total amount of cyclovirboxicin D and its related substances, and cyclovirboxicin D is only one of the alkaloids, and this detection method can also Unable to accurately determine the true content of Cycloviral Buxicine D
The preparations Huangyangning dripping pills and Huangyangning powder injections produced with Buyangning as the original drug are currently also using acid dye colorimetry to measure the content of cyclovirbuxuxin D contained in them, that is, the measured is also the total alkaloid content of alkaloids, which cannot Accurate Determination of the Content of Cyclovitamin D in it
Because of the disadvantages of poor specificity in the above-mentioned determination methods, many researchers are actively looking for highly specific determination methods, especially high-performance liquid chromatography, such as pre-column derivatization high-performance liquid chromatography, end-absorption high-performance liquid chromatography, high-efficiency Liquid phase-mass spectrometry, etc., but these methods all have such and such shortcomings
Using pre-column derivatization high-performance liquid chromatography to determine the content of cyclovirboxicin D, it is necessary to make cyclovirboxicin D generate a new compound through a chemical reaction before sample injection, and then convert the content of the new compound into a ring The content of vitamin Buxicine D, so it is an indirect determination method. Ester is a harmful liquid and is used as a lachrymator. It is irritating, photosensitive, and sensitive to light and humidity. It must be stored after being filled with nitrogen. Conventional storage is prone to failure. Long-term use will cause great harm to the human body, so it is not recommended Suitable for use as a derivatization reagent to determine the content of Cyclovirubuxin D
Application number: 200310106142.8, publication number: the invention patent of CN1540339A adopts end-absorption high-performance liquid chromatography to measure the content of Cyclovirine buxuxin D, but this method adopts aminopropylsilane bonded silica gel as filler, and its versatility is not strong; and The terminal absorbance at 210nm is used for detection, and the selectivity is not strong. Therefore, the pretreatment operation is cumbersome when measuring the cyclovirbuxuxin D in Huangyangning tablets, Huangyangning dripping pills and Huangyangning powder injections.
In addition, there are also literatures using high-performance liquid chromatography-mass spectrometry. This method has poor precision and is expensive. At present, people basically do not use this method as a method for the determination of Cyclovitamin D.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
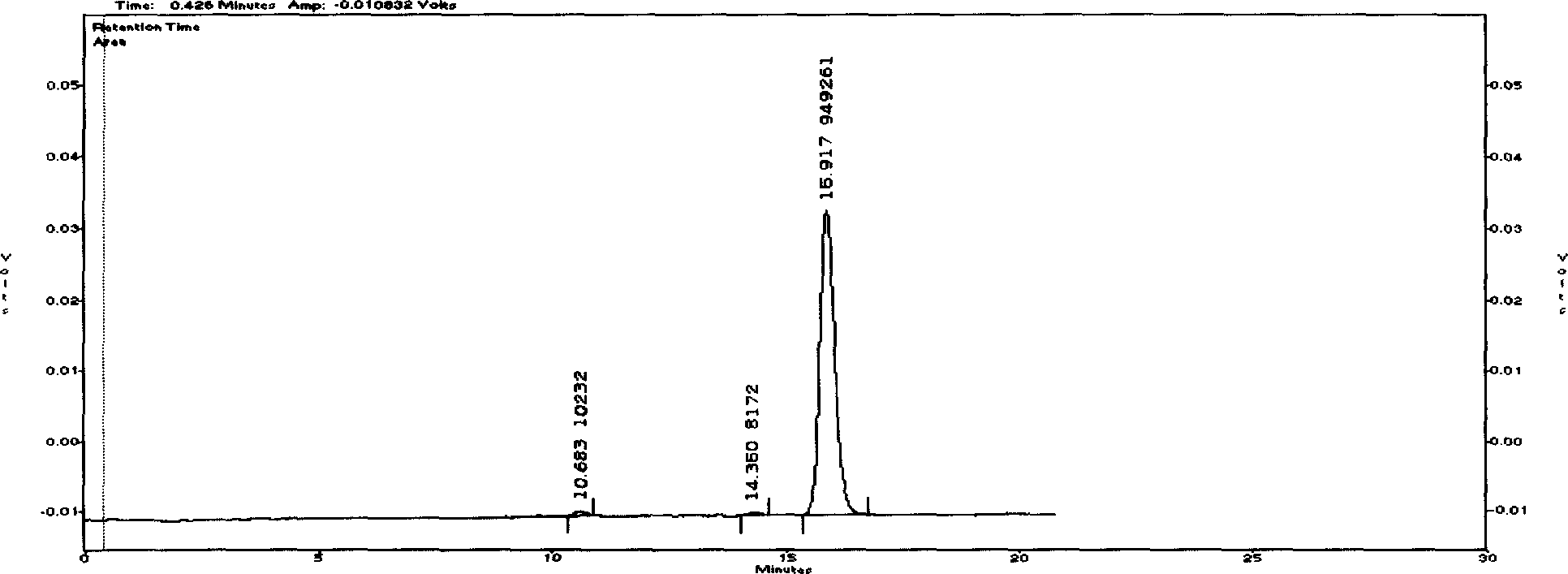
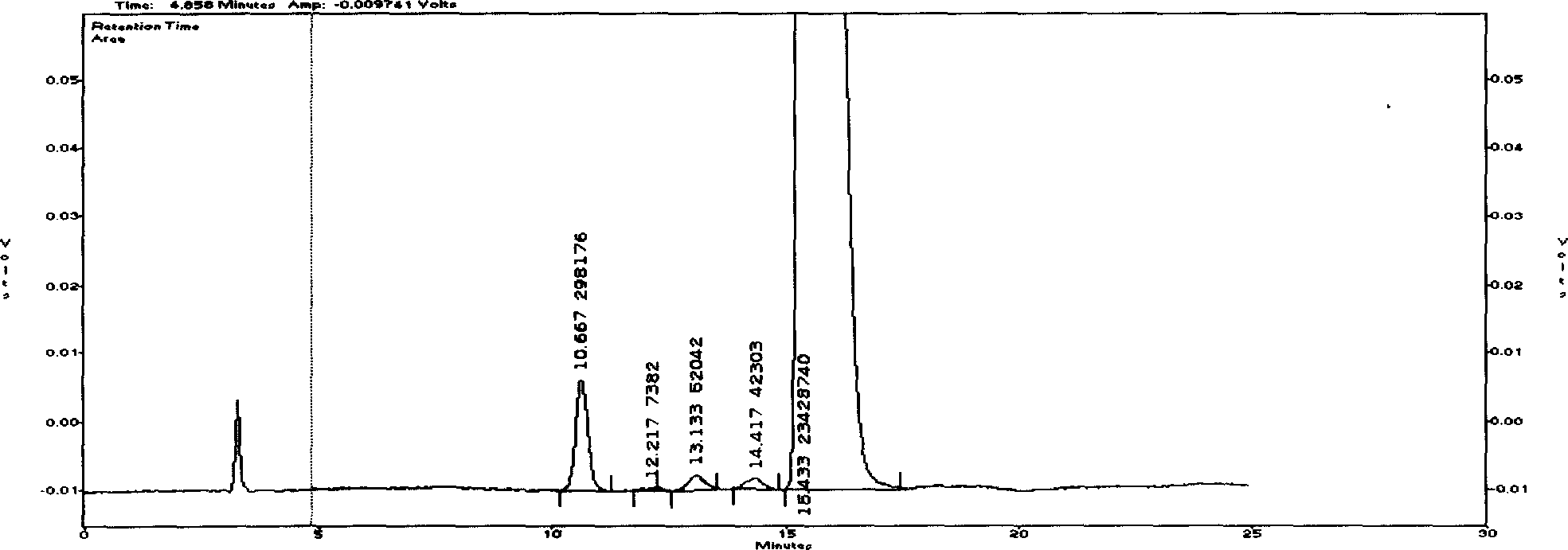
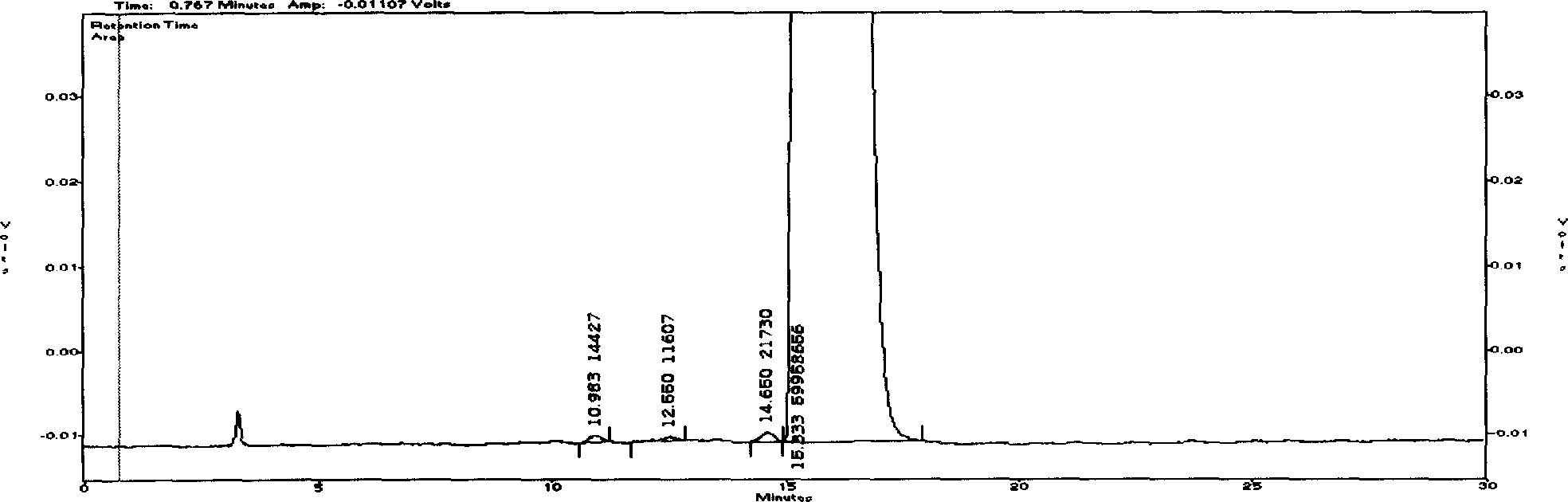
The invention discloses a Cyclovirobuxine-D raw material drug and high efficiency liquid chromatography mensuration for Cyclovirobuxine-D content in different kinds of preparation, and high efficiency liquid chromatography for impurity testing in Cyclovirobuxine-D. The fixed phase in chromatographic condition uses octadecyl silane linkage silica gel or octyl silane linkage silica gel as filling material; The mobile phase adopt formate (ammonium formate) damping fluid-methanol system, acetic acid (ammonium acetate) damping fluid-methanol system, formate (ammonium formate) damping fluid-acetonitrile system or acetic acid (ammonium acetate) damping fluid-acetonitrile system; Evaporation light scattering detector (ELSD) detects drift tube temperature; drift tube temperature: 20-150 degree centigrade;and gas flow rate: 0.1L / min- 6.0L / min, split-flow or not split stream sampling. The method is easy to operate, timesaving, and has strong expert attribute. It could effectively divide some kind of alkaloids that has with Cyclovirobuxine-D in Cyclovirobuxine-D raw material drug. Thus, it is able to accurately measure their content. The method could also detecting impurity in Cyclovirobuxine-D. The invention could also measure content degree of homogeneity of Cyclovirobuxine-D in boxwood-nin piece, boxwood-nin drop pill, and boxwood-nin powder pin. And it commendably satisfies the request of pharmacopoeia content and content degree of homogeneity testing.
Description
Chromatographic Determination Method of Cyclovirboxicine D Content in Cyclovirboxicine D Raw Materials and Its Preparations technical field The present invention relates to a high-performance liquid chromatography method for determining the content of cyclovitamin D in the bulk drug and various preparations thereof (such as Huangyangning tablets, Huangyangning dripping pills, Huangyangning powder injections, etc.) and cyclovitamin D. High performance liquid chromatography for impurity check in D. In particular it involves the use of high performance liquid chromatography with detection using an evaporative light scattering detector (ELSD). Background technique Buxus mincrophylla Sieb.etZucc.var.sinicaRehd.etWils. and its whole plants belonging to the family Buxus family, the alkaloids obtained by extraction and refining, if the main component of Buxus D is more than 70%, it is Buxus Star D API, also known as Huang Yang Ning. At present, the commonly used assay methods f...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): G01N30/88G01N30/02G01N30/74G01N30/06G01N30/60
Inventor 李伟魏峰
Owner TIANJIN TASLY PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com