Cyclovirobuxine D raw medicine and method for determining its content in preparation by chromatography
The invention relates to a technology of cyclovir buxus and raw materials, which is applied to the detection of an evaporative light scattering detector. , In the field of high performance liquid chromatography, it can solve the problems of cumbersome operation, easy storage failure, poor specificity, etc., and achieve the effect of simple operation method, strong versatility and simple method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
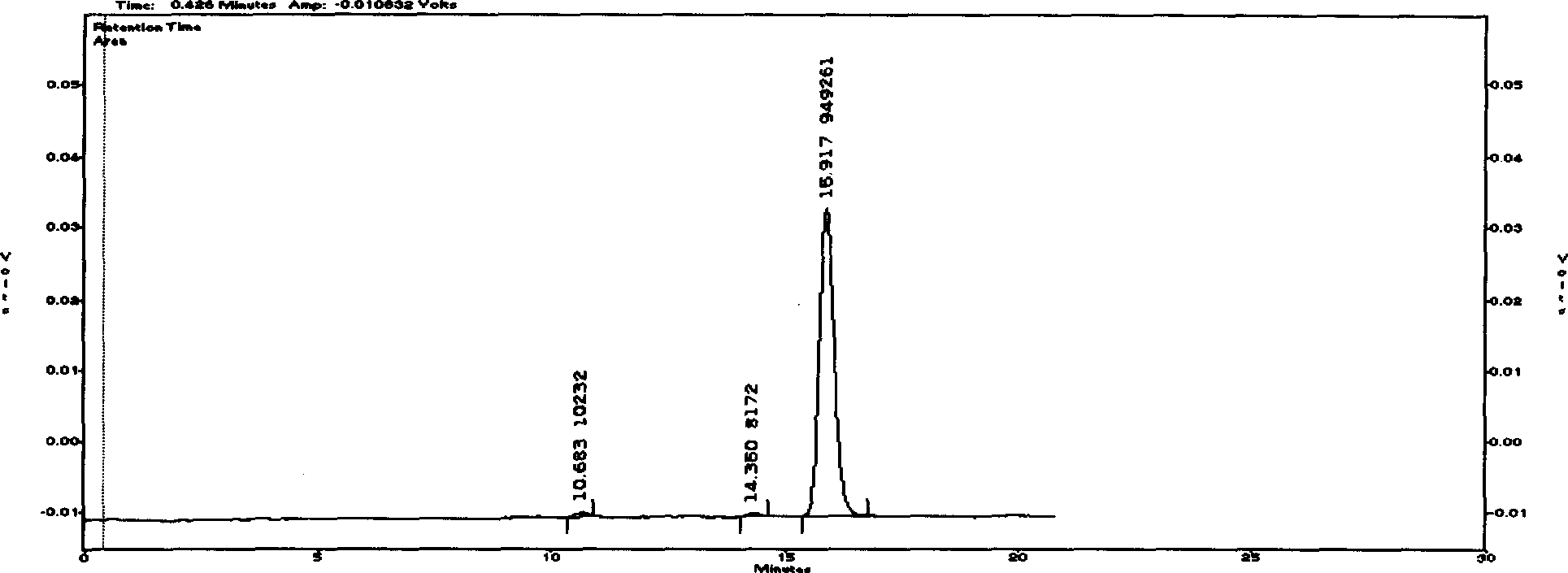
[0045] Huanwei Buxus D raw material medicine is commercially available (produced by Henan Longdu Pharmaceutical Factory)
[0046] Chromatographic conditions and system adaptability The stationary phase uses octadecylsilane bonded silica gel as a filler; ammonium formate buffer solution (take 15.0 g of ammonium formate, add 10 ml of formic acid, add water to dissolve and dilute to 1000 ml)-methanol (volume ratio of 62:38) is the mobile phase; the flow rate is 0.8ml / min; evaporative light scattering detector: the drift tube temperature is 100°C, and the gas flow rate is 2.7L / min; the column temperature is 30°C; in the chromatogram of the test solution, The D peak of Cyclovitamin and other alkaloid peaks should be separated by baseline; the number of theoretical plates should be no less than 2000 based on the calculation of Cyclovitamin D peak.
[0047] Preparation of Reference Substance Solution Use phosphorus pentoxide as a desiccant, and dry at 60°C under reduced pressure to c...
Embodiment 2
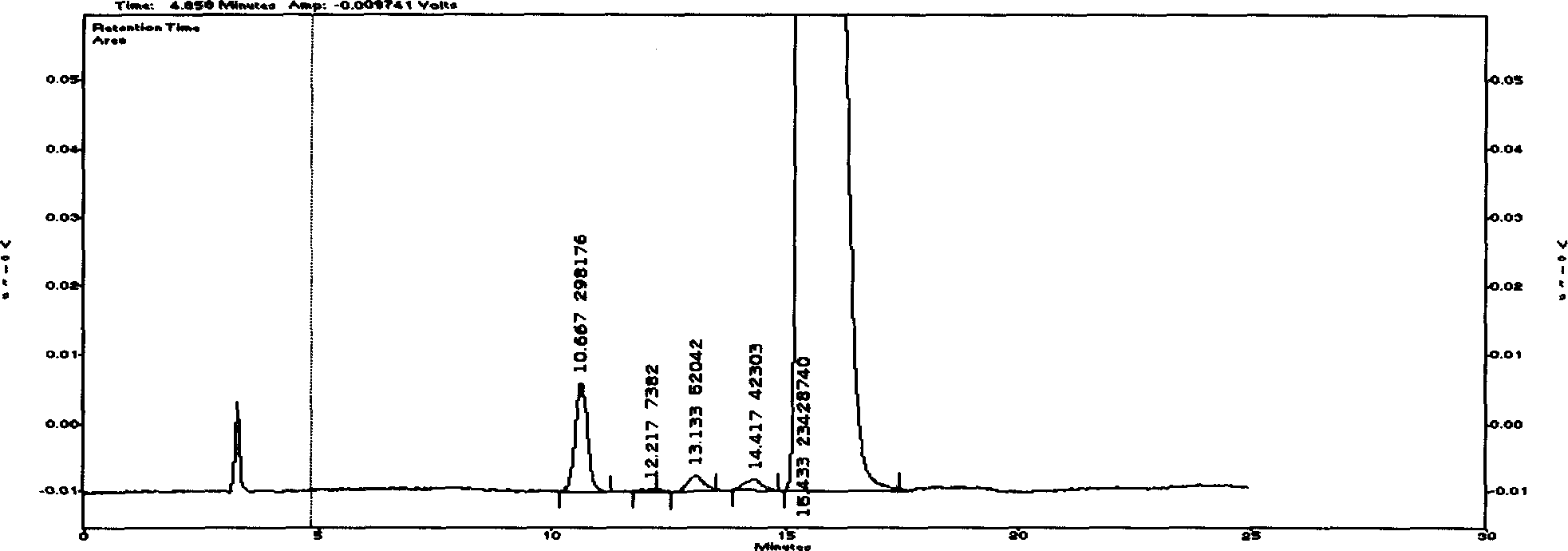
[0056] Embodiment two (cyclovir buxicine D reference substance purity inspection)
[0057] Cyclovitamin D reference substance (purchased from China Institute for Pharmaceutical and Biological Products Inspection, batch number 888-200001)
[0058] Chromatographic conditions and system adaptability The stationary phase uses octadecylsilane bonded silica gel as a filler; ammonium formate buffer solution (take 15.0 g of ammonium formate, add 10 ml of formic acid, add water to dissolve and dilute to 1000 ml)-methanol (volume ratio of 62:38) is the mobile phase; the flow rate is 0.8ml / min; evaporative light scattering detector: the drift tube temperature is 100°C, and the gas flow rate is 2.7L / min; the column temperature is 30°C; in the chromatogram of the test solution, The D peak of Cyclovitamin and other alkaloid peaks should be separated by baseline; the number of theoretical plates should be no less than 2000 based on the calculation of Cyclovitamin D peak.
[0059] Preparatio...
Embodiment 3
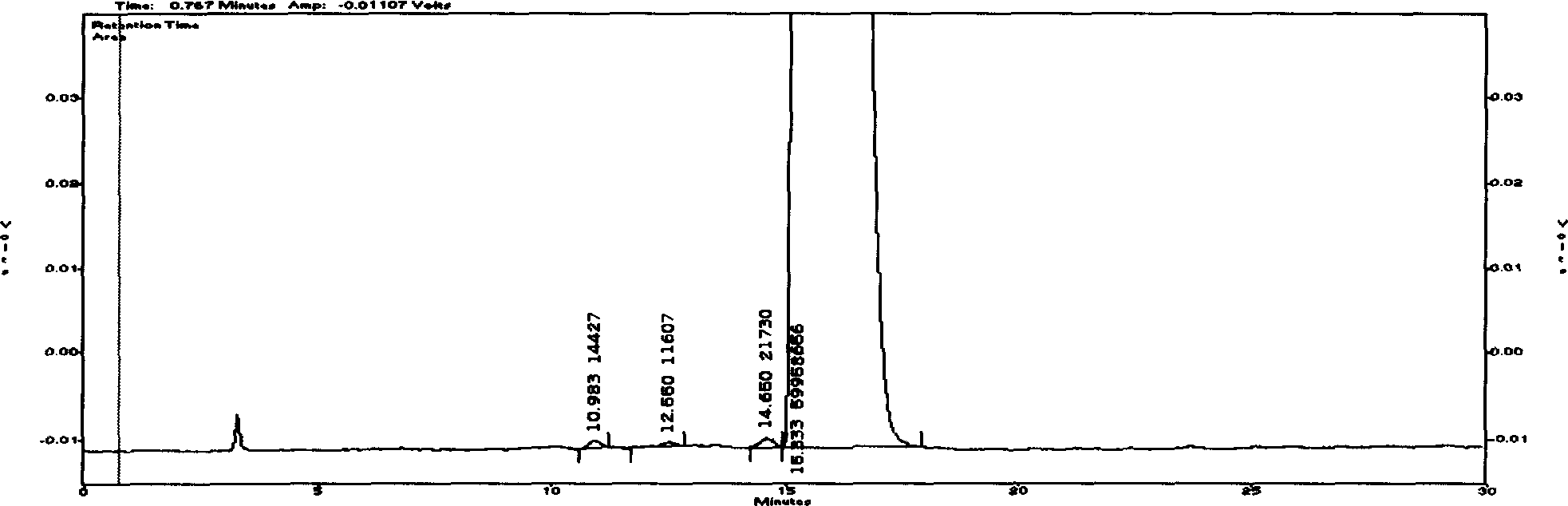
[0063] Embodiment three (purity inspection of the raw material of refined cyclovir buxicine D)
[0064] Refined Huowei Buxus D raw material (provided by Henan Kangli Pharmaceutical Technology Development Co., Ltd.)
[0065] Chromatographic conditions and system adaptability The stationary phase uses octadecylsilane bonded silica gel as a filler; ammonium formate buffer solution (take 15.0 g of ammonium formate, add 10 ml of formic acid, add water to dissolve and dilute to 1000 ml)-methanol (volume ratio of 63:37) is mobile phase; Flow rate is 0.8ml / min; Evaporative light scattering detector: drift tube temperature is 100 ℃, and gas flow rate is 2.7L / min; Column temperature is 30 ℃; In the chromatogram of need testing solution, The D peak of Cyclovitamin and other alkaloid peaks should be separated by baseline; the number of theoretical plates should be no less than 2000 based on the calculation of Cyclovitamin D peak.
[0066] Preparation of the test solution Take 10 mg of re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com