A kind of preparation method of Cyclovitamin D hydrochloride
A technology of cyclovir buxus and hydrochloride, which is applied in the field of medicine and chemical industry, can solve the problems of cumbersome column chromatography method, low yield, unsuitable for industrial production, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
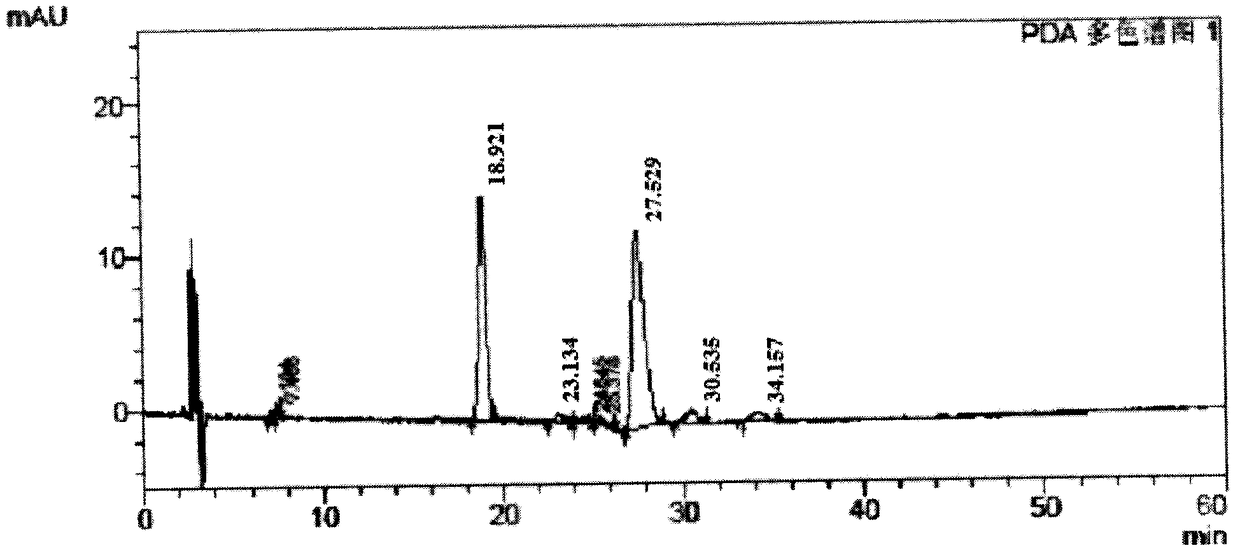
[0038] Using the commercially available product of Cycloviral Buxusin D (Nanjing Shizhou Pharmaceutical Technology Co., Ltd.) as a raw material, the method disclosed in CN1594355A was used for refining to obtain the secondary crystal product of Cycloviral Buxusin D, and the purity was measured by TLC chromatographic system and HPLC respectively. .
[0039] TLC chromatographic system: thin-layer plate: silica gel G thin-layer plate; developer: chloroform: hexane: methanol: ammonia water = 2.5: 4: 1: 0.1; chromogen; dilute potassium iodide reagent; sample volume; 100 ~250 μg). The measurement results show that the TLC purity of the secondary crystalline product of Cyclovirubuxin D prepared by the method disclosed in CN1594355A is 96%.
[0040] HPLC system: Chromatographic column: Yuexu C18 (250mm×4.6mm, 5μm), mobile phase A: 0.01M sodium heptanesulfonate and 0.01M KH 2 PO 4 Equivalent mixed solution (containing 0.2% triethylamine, adjusted pH to 3.50 with phosphoric acid), mo...
Embodiment 2
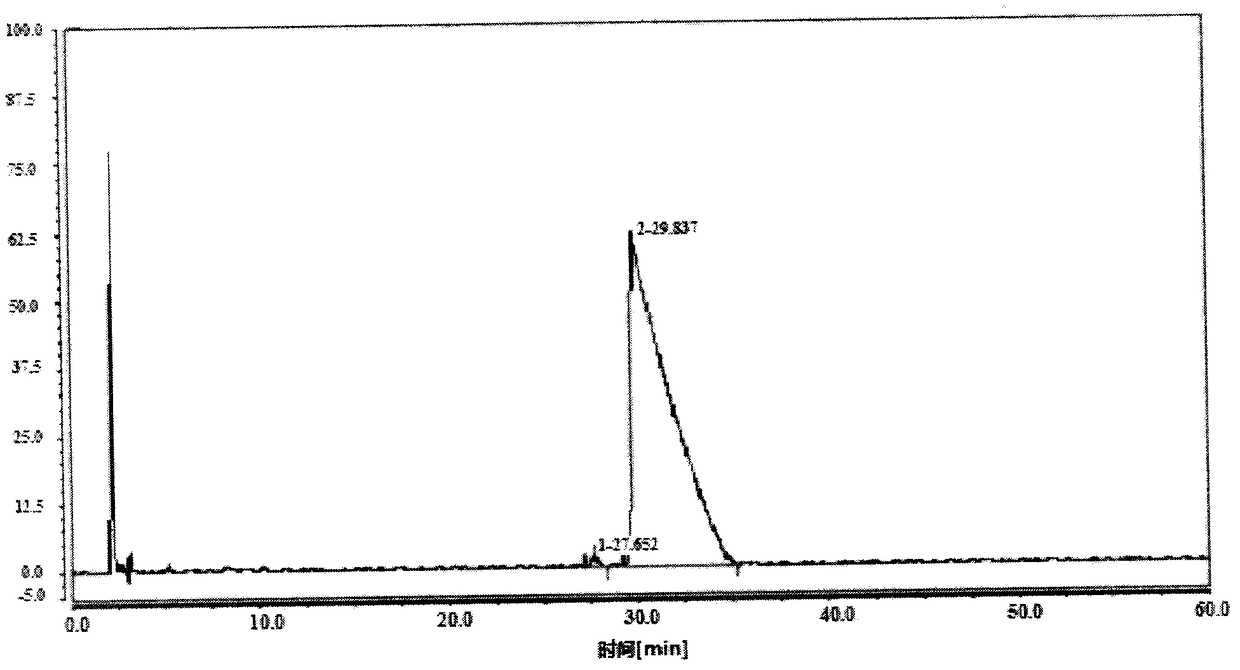
[0047] Take the commercially available Huowei Buxus D, add 20 times the volume (W / V) of methanol, heat and stir until reflux (65°C), dissolve, and reflux for 15 minutes after dissolution, stop heating, slowly and naturally cool down, and stir to crystallize. Referring to this method, the crystallization was repeated four times to obtain four crystallization products. The mother liquor is used repeatedly, and after four times of crystallization, the yield reaches about 70%. The four crystallization results are as follows:
[0048] Table 3. Crystallization results of commercially available Cycloviral Buxicin D in methanol
[0049]
[0050] As shown in Table 3, the data in the table are the crude product and the 1st to 4th crystallization of the crude product in sequence. The first crystallization effect is particularly remarkable. The purity of Cyclovitamin D rose from 54.51% to 91.27%. However, the effect of the subsequent three crystallizations was not very signifi...
Embodiment 3
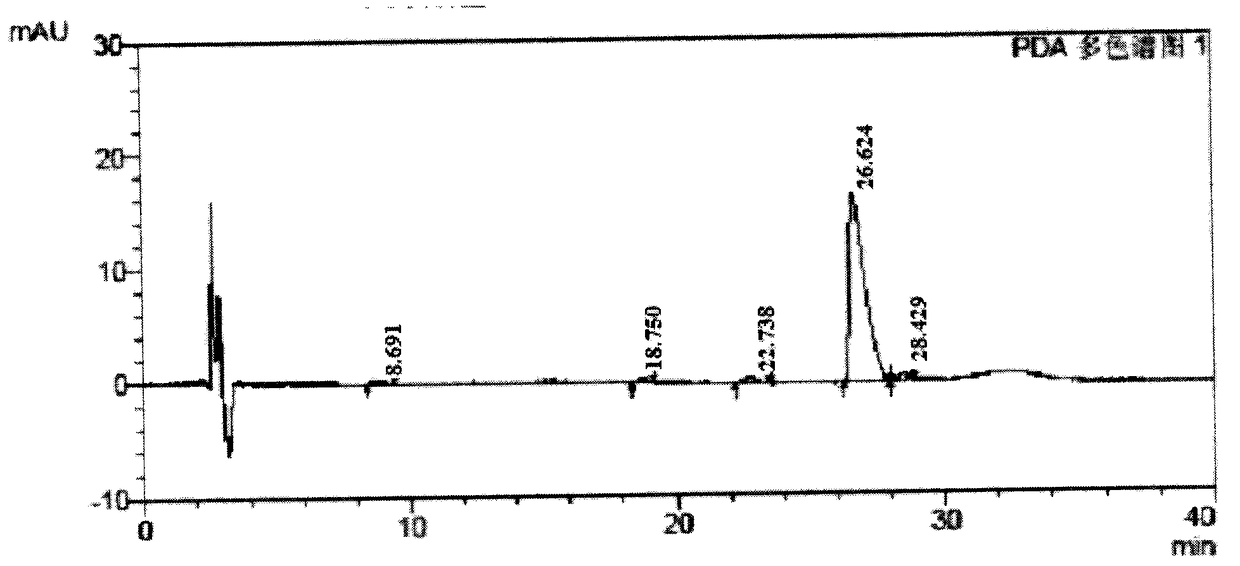
[0052] The tertiary crystalline product of Cyclovirubicin D in Example 2 is used as a raw material, as a sample to be formed into a salt, and its liquid phase purity is about 97%.
[0053] Get 30g of the third crystallization product of cyclovir buxicine D and divide it into three parts on average, dissolve them in 40 times of volume (W / V) water, 40 times of volume (W / V) methanol and 40 times of volume (W / V) methylene chloride, The salt-forming reaction was carried out at room temperature. Then add 2eq 1M dilute hydrochloric acid dropwise at room temperature and stir to form a salt. After water is used as solvent to form a salt, crystals cannot be precipitated or crystals are less at room temperature, and the temperature is lowered to about 4°C to crystallize; after methanol is used as a solvent to form a salt, the product and methanol are in the form of a paste, which is difficult to filter; Methane is used as a solvent. After the salt-forming reaction, the product is direct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com